Chloroquine and its derivatives in the management of COVID-19: A scoping review
Abstract
Introduction: Recently, researchers from China and France reported on the effectiveness of chloroquine and hydroxychloroquine for the inhibition of SARS-CoV-2 viral replication in vitro. Timely dissemination of scientific information is key in times of pandemic. A systematic review of the effect and safety of these drugs on COVID-19 is urgently needed.
Objective: To map published studies until March 25, 2020, on the use of chloroquine and its derivates in patients with COVID-19.
Materials and methods: We searched on PubMed, Embase, Lilacs, and 15 registries from the World Health Organization’s International Clinical Trials Registry Platform for theoretical and empirical research in English, Spanish, Italian, French, or Portuguese until March 25, 2020, and made a narrative synthesis of the results.
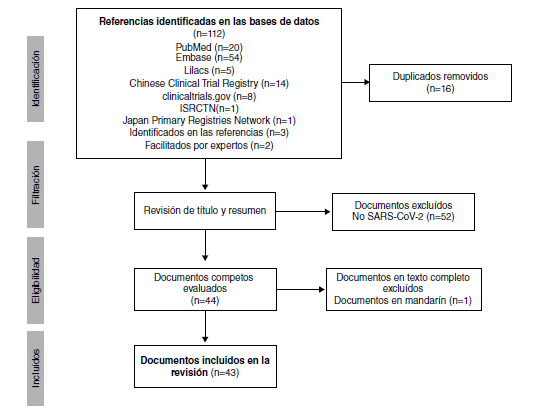
Results: We included 19 records and 24 trial registries (n=43) including 18,059 patients. China registered 66% (16/24) of the trials. Nine trials evaluate chloroquine exclusively and eight hydroxychloroquine. The records are comments (n=9), in vitro studies (n=3), narrative reviews (n=2), clinical guidelines (n=2), as well as a systematic review, an expert consensus, and a clinical trial.
Conclusions: One small (n=26), non-randomized, and flawed clinical trial supports hydroxychloroquine use in patients with COVID-19. There is an urgent need for more clinical trial results to determine the effect and safety of chloroquine and hydroxychloroquine on COVID-19.
Downloads
References
World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19. [Fecha de consulta: 23 de marzo de 2020]. Disponible en: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
World Health Organization. Coronavirus disease 2019. [Fecha de consulta: 8 de abril de 2020]. Disponible en: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67-9. https://doi.org/10.1016/S1473-3099(06)70361-9
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-66. https://doi.org/10.1056/NEJMoa030781
Keyaerts E, Vijgen L, Maes P, Neyts J, Ranst M Van. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264-8. https://doi.org/10.1016/j.bbrc.2004.08.085
Yu IT-S, Qiu H, Tse LA, Wong TW. Severe acute respiratory syndrome beyond Amoy gardens: completing the incomplete legacy. Clin Infect Dis. 2014;58:683-6. https://doi.org/10.1093/cid/cit797
Colson P, Rolain J-M, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923. https://doi.org/10.1016/j.ijantimicag.2020.105923
Keyaerts E, Li S, Vijgen L, Rysman E, Verbeeck J, Van Ranst M, et al. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother. 2009;53:3416-21. https://doi.org/10.1128/AAC.01509-08
de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875-84. https://doi.org/10.1128/AAC.03011-14
Rolain J-M, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297-308. https://doi.org/10.1016/j.ijantimicag.2007.05.015
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-71. https://doi.org/10.1038/s41422-020-0282-0.
Devaux CA, Rolain J-M, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;105938. https://doi.org/10.1016/j.ijantimicag.2020.105938
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. https://doi.org/10.1016/S0140-6736(20)30183-5
Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683-4. https://doi.org/10.1016/S0140-6736(20)30361-5
Ministerio de Salud y Protección Social - República de Colombia. Hidroxicloroquina y cloroquina se podrán usar para tratamiento de covid – 19. 2020 [Fecha de consulta: 8 de abril de 2020]. Disponible en: https://www.minsalud.gov.co/Paginas/Hidroxicloroquina-y-cloroquina-se-podran-usar-para-tratamiento-de-covid-–-19.aspx
Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177:104762. https://doi.org/10.1016/j.antiviral.2020.104762
Song P, Karako T. COVID-19: Real-time dissemination of scientific information to fight a public health emergency of international concern. Biosci Trends. 2020;14:1–2. https://doi.org/10.5582/bst.2020.01056
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19-32. https://doi.org/10.1093/geront/gnz021
Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. https://doi.org/10.1186/1748-5908-5-69
World Health Organization. WHO Registry Network. 2020 [Fecha de consulta: 25 de marzo de 2020]. Disponible en: https://www.who.int/ictrp/network/primary/en/
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4
Grudniewicz A, Nelson M, Kuluski K, Lui V, Cunningham H V., X Nie J, et al. Treatment goal setting for complex patients: protocol for a scoping review. BMJ Open. 2016;6:e011869. https://doi.org/10.1136/bmjopen-2016-011869.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467. https://doi.org/10.7326/M18-0850
Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72-73. https://doi.org/10.5582/bst.2020.01047
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa237
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. https://doi.org/10.1038/s41421-020-0156-0
Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949. https://doi.org/10.1016/j.ijantimicag.2020.105949
Wilson P. COVID-19, Hydroxychloroquine, and the Death of Evidence-Based Medicine. The Methods Man. 2020 [Fecha de consulta: 2 de abril de 2020]. Disponible en: https://www.methodsman.com/blog/covid-19-evidence
Hinton DM. US Food & Drug Administration Emergency Use Autorization for chloroquine phosphate and hydroxychloroquine sulfate use for the treatment of COVID-19. 2020. p. 1–8. [Fecha de consulta: 2 de abril de 2020]. Disponible en: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f398f8a9-92f3-47cb-81c2-6078806a464d
U.S. Department of Health and Human Services (HHS). HHS accepts donations of medicine to Strategic National Stockpile as possible treatments for COVID-19 patients | HHS.gov. News. 2020 [Fecha de consulta: 2 de abril de 2020]. Disponible en: https://www.hhs.gov/about/news/2020/03/29/hhs-accepts-donations-of-medicine-to-strategic-national-stockpile-as-possible-treatments-for-covid-19-patients.html#
O’Brien N, Hong QN, Law S, Massoud S, Carter A, Kaida A, et al. Health system features that enhance access to comprehensive primary care for women living with hiv in high-income settings: a systematic mixed studies review. AIDS Patient Care STDS. 2018;32:129-48. https://doi.org/10.1089/apc.2017.0305
Alvarado-Castro V, Paredes-Solís S, Nava-Aguilera E, Morales-Pérez A, Alarcón-Morales L, Balderas-Vargas NA, et al. Assessing the effects of interventions for Aedes aegypti control: systematic review and meta-analysis of cluster randomised controlled trials. BMC Public Health. 2017;17(Supl.1):384. https://doi.org/10.1186/s12889-017-4290-z
Shalabi D, Mitchell S, Andersson N. Review of Gender Violence Among Arab Immigrants in Canada: Key Issues for Prevention Efforts. J Fam Violence. 2015;30:817–25. https://doi.org/10.1007/s10896-015-9718-6
Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7. https://doi.org/10.1186/1471-2288-7-10
Pimentel J, Arias A, Ramírez D, Molina A, Chomat A-M, Cockcroft A, et al. Game-based learning interventions to foster cross-cultural care training: a scoping review. Games Health J. 2020. https://doi.org/10.1089/g4h.2019.0078.
Pimentel J, Ansari U, Omer K, Gidado Y, Baba MC, Andersson N, et al. Factors associated with short birth interval in low- and middle-income countries: a systematic review. BMC Pregnancy Childbirth. 2020;20:156. https://doi.org/10.1186/s12884-020-2852-z
Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13:141-6. https://doi.org/10.1097/XEB.0000000000000050.
Some similar items:
- Amanda Maestre, Jaime Carmona-Fonseca, Amanda Maestre, Alta frecuencia de mutaciones puntuales en pfcrt de Plasmodium falciparum y emergencia de nuevos haplotipos mutantes en Colombia , Biomedica: Vol. 28 No. 4 (2008)
- Angélica Knudson, Rubén Santiago Nicholls, Ángela Patricia Guerra, Ricardo Sánchez, Clinical profiles of patients with uncomplicated Plasmodum falciparum malaria in northwestern Colombia , Biomedica: Vol. 27 No. 4 (2007)
- Paula Montoya, Alberto Tobón, Silvia Blair, Jaime Carmona, Amanda Maestre, Polymorphisms of the pfmdr1 gene in field samples of Plasmodium falciparum and their association with therapeutic response to antimalarial drugs and severe malaria in Colombia , Biomedica: Vol. 27 No. 2 (2007)
- Jaime Carmona-Fonseca, Gonzalo Álvarez, Silvia Blair, Plasmodium vivax malaria: treatment of primary attacks with primaquine, in three different doses, and a fixed dose of chloroquine, Antioquia, Colombia, 2003-2004 , Biomedica: Vol. 26 No. 3 (2006)
- Jorge Rivera, Ladys Sarmiento, Edgar Parra, Gabriel Toro, Marcela Neira, Jairo Méndez, Juliana Barbosa, María Leonor Caldas, Morphological changes in lung tissue of victims associated with the 2009 A H1N1/v09 influenza pandemic in Colombia , Biomedica: Vol. 31 No. 3 (2011)
- Manuel Alberto Pérez, Liliana Jazmín Cortés, Ángela Patricia Guerra, Angélica Knudson, Carlos Usta, Rubén Santiago Nicholls, Efficacy of the amodiaquine+sulfadoxine-pyrimethamine combination and of chloroquine for the treatment of malaria in Córdoba, Colombia, 2006 , Biomedica: Vol. 28 No. 1 (2008)
- Arletta Añez, Dennis Navarro-Costa, Omar Yucra, Cecilia Garnica, Viviana Melgar, Manuel Moscoso, Ricardo Arteaga, Gladys Nakao, Therapeutic response of Plasmodium vivax to chloroquine in Bolivia , Biomedica: Vol. 32 No. 4 (2012)
- Lorenzo Cáceres, José Rovira, Rolando Torres, Arsenio García, José Calzada, Manuel De La Cruz, Characterization of Plasmodium vivax malaria transmission at the border of Panamá and Costa Rica , Biomedica: Vol. 32 No. 4 (2012)
- Silvia Blair, Mary Luz López, Juan Gabriel Piñeros, Tania Alvarez, Alberto Tobón, Jaime Carmona, Therapeutic efficacy of 3 treatment protocols for non-complicated Plasmodium falciparum malaria, Antioquia, Colombia, 2002. , Biomedica: Vol. 23 No. 3 (2003)
- Eliana Arango, Tania Alvarez, Jaime Carmona, Silvia Blair, Gametocyte levels in response to differing malaria treatments in two municipalities of Colombia. , Biomedica: Vol. 24 No. 1 (2004)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























