Skeletal malformations and growth disturbances in fetuses of mild diabetic rats
Abstract
Introduction: Currently, diabetes mellitus represents one of the medical conditions that more frequently complicates pregnancy affecting the fetus’s growth and development.
Objective: To determine the skeletal malformations and growth alterations in fetuses of diabetic Wistar rats.
Materials and methods: We used a neonatally streptozotocin-induced mild diabetes model (STZ 100 mg/kg body weight - subcutaneously) in Wistar rats. In adulthood, healthy and diabetic rats were mated with healthy males of the same age and strain. On day 20 of gestation, a cesarean was performed under anesthesia. Fetuses were removed, weighed, and classified as small (SPA), adequate (APA), and large (LPA) for the gestational age. Selected fetuses were processed for skeletal anomaly and ossification sites analysis.
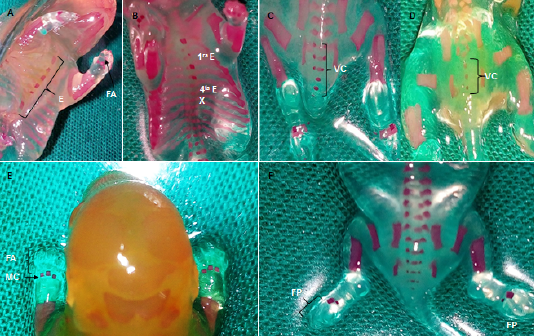
Results: In the offspring of diabetic rats, there was a higher percentage of fetuses classified as small or large and a lower percentage of fetuses with adequate weight; the fetal weight mean was lower and there were fewer sites of ossification. Alterations were observed in the ossification of the skull, sternum, spine, ribs and fore and hind limbs; and also, there was a direct correlation between fetal weight and ossification degree. There were congenital malformations associated with fusion and bifurcation of the ribs, as well as changes indicative of hydrocephaly, such as the dome shape of the skull, a wide distance between parietals, and the width of the anterior and posterior fontanels.
Conclusion: Moderate diabetes during pregnancy alters fetal growth and development with macrosomia and intrauterine growth restriction, as well as skeletal malformations.
Downloads
References
World Health Organization. Global Report on Diabetes. Geneva: WHO; 2016.
International Diabetes Federation. Diabetes Atlas. Brussels-Belgium: IDF; 2019.
Kelstrup L, Bytoft B, Hjort L, Houshmand-Oeregaard A, Mathiesen E, Damm P. Diabetes in pregnancy. In: Lapolla A, Metzger BE, editors. Gestational diabetes. 28 edition. New York: Basel, Karger; 2020. p. 201-22. https://doi.org/10.1159/000480176
Jean-Baptiste A, Simeoni U. Offspring of mothers with hyperglycemia in pregnancy: Shortterm consequences for newborns and infants. In: Lapolla A, Metzger BE, editors. Gestational diabetes. New York: Basel, Karger; 2020. p. 194-200. https://doi.org/10.1159/000480175
Gutaj P, Wender-Ozegowska E. Diagnosis and management of IUGR in pregnancy complicated by type 1 diabetes mellitus. Curr Diab Rep. 2016;16:1-9. https://doi.org/10.1007/s11892-016-0732-8
Wentzel P, Eriksson U. Embryopathy and diabetes. In: Lapolla A, Metzger BE, editors. Gestational diabetes. New York: Basel, Karger; 2020. p. 132-44. https://doi.org/10.1159/000480170
MINSAP. Ministerio de Salud Pública de Cuba. Dirección de Registros Médicos y Estadísticas de Salud. Anuario estadístico de salud 2019. La Habana: Ministerio de Salud Pública; 2020.
Parodi K, Jose S. Diabetes y embarazo. Rev Fac Cienc Méd. 2016;1:27-35.
Jawerbaum A, White V. Animal models in diabetes and pregnancy. Endocr Rev. 2010;31:680-701. https://doi.org/10.1210/er.2009-0038
Eriksson UJ, Wentzel P. The status of diabetic embryopathy. Ups J Med Sci. 2016;121:96-112. https://doi.org/10.3109/03009734.2016.1165317
Jawerbaum A, White V. Review on intrauterine programming: Consequences in rodent models of mild diabetes and mild fat overfeeding are not mild. Placenta. 2017;52:21-32. https://doi.org/10.1016/j.placenta.2017.02.009
Friedman JE. Obesity and gestational diabetes mellitus pathways for programming in mouse, monkey, and man. Diabetes Care. 2015;38:1402-11. https://doi.org/10.2337/dc15-0628
Bequer L, Gómez T, Molina J, Álvarez A, Chaviano C, Clapés S. Experimental diabetes impairs maternal reproductive performance in pregnant Wistar rats and their offspring. Syst Biol Reprod Med. 2018;64:7. https://doi.org/10.1080/19396368.2017.1395928
Bequer L, Gómez T, Molina JL, Artiles D, Bermúdez R, Clapés S. Acción diabetogénica de la estreptozotocina en un modelo experimental de inducción neonatal. Biomédica. 2016;26:230-8. https://doi.org/10.7705/biomedica.v36i2.2686
Gómez T, Bequer L, Sánchez C, de la Barca M, Muro I, Reyes MA, et al. Inducción neonatal de hiperglucemias moderadas: indicadores metabólicos y de estrés oxidativo en ratas adultas. Rev ALAD. 2014;4:148-57.
National Institute of Health. Guide for the care and use of laboratory animals. Washington, D. C.: National Academies Press; 2011.
Soulimane-Moktari N, Guermouche B, Yessoufou A, Saker M, Moutairou K, Hichami A. Modulation of lipid metabolism by n-3 polyunsaturated fatty acids in gestational diabetic rats and their macrosomic offspring. Clin Sci. 2005;109:287-95. https://doi.org/10.1042/CS20050028
Staples RE, Schnell VL. Refinements en rapid clearing techinc in the KOH-alizarine red S method for fetal bone. Stain Technology. 1964;39:61-3.
Damasceno DC, Kempinas WG, Volpato GT, Consoni M, Rudge MVC, Paumgartten FJR. Anomalias congênitas: Estudos experimentais. 1a edicion. Belo Horizonte: Coopmed; 2008. p. 102.
Aliverti V, Bonanomi L, Giavini E, Leone VG, Mariani L. The extent of fetal ossification as an index of delayed development in teratogenic studies on the rat. Teratology. 1979;20:237-42. https://doi.org/10.1002/tera.1420200208
Saito FH, Damasceno DC, Dallaqua B, Moreno I, Rudge MVC, De Mattos I. Heat shock protein production and immunity and altered fetal development in diabetic pregnant rats. Cell Stress Chaperones. 2013;18:25-33. https://doi.org/10.1007/s12192-012-0353-3
Iessi IL, Bueno A, Sinzato YK, Taylor KN, Rudge MV, Damasceno DC. Evaluation of neonatally-induced mild diabetes in rats: Maternal and fetal repercussions. Diabetol Metab Syndr. 2010;37. https://doi.org/10.1186/1758-5996-2-37
Cunningham FG, Leveno KL, Bloom SL, Spong CY, Dashe J, Hoffman BL. Williams Obstetrics. 25 edition. New York,United States: McGraw-Hill Education; 2018. p. 1344.
Elizabeth KE, Ashwin DA, Sobhakumar S, Sujatha TL. Outcome of large- and small-forgestational-age babies born to mothers with pre-pregnancy and gestational diabetes mellitus versus without diabetes mellitus. Indian J Child Health. 2018;5:592-6. https://doi.org/10.32677/IJCH.2018.v05.i09.011
Huynh J, Dawson D, Roberts D, Bentley-Lewis R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta. 2015;36:101-14. https://doi.org/10.1016/j.placenta.2014.11.021
Raymond GY, Robert M. Test methods for assessing female reproductive and developmental toxicology. En: Hayes WA, Kruger CL. Hayes' principles and methods of toxicology. 6th edition. London: CRC Press; 2014. p. 1639-712. https://doi.org/10.1201/b17359
Damasceno DC, Dallaqua B, Iessi IL, Volpato GT, Campos KE. Impact of maternal overnutrition during pregnancy on maternal oxidative stress and fetal skeletal/visceral anomalies of the rats. J Nutr Disorders Ther 2016;6:1-5. https://doi.org/10.4172/2161-0509.1000185
Saito FH, Damasceno DC, Kempinas WG, Morceli G, Sinzato YK, Taylor KN, et al. Repercussions of mild diabetes on pregnancy in Wistar rats and on the fetal development. Diabetol Metab Syndr. 2010;2:8. https://doi.org/10.1186/1758-5996-2-26
Lewis EM. Reproductive toxicology: historical control data in rats. Pennsylvania: Charles River Laboratories; 2017. p .54.
Loeken MR. Mechanisms of congenital malformations in pregnancies with pre-existing diabetes. Curr Diab Rep. 2020;20:12. https://doi.org/10.1007/s11892-020-01338-4
Zabihi S, Loeken MR. Understanding diabetic teratogenesis: Where are we now and where are we going? Birth Defects Res A Clin Mol Teratol. 2018;88:779-90. https://doi.org/10.1002/bdra.20704
Castori M. Diabetic embryopathy: A developmental perspective from fertilization to adulthood. Mol Syndromol. 2013;4:74-86. https://doi.org/10.1159/000345205
Some similar items:
- Jorge Hernán Montoya, Olga Lucía Morales, Four cases of Jarcho-Levin’s syndrome in the province of Antioquia, Colombia , Biomedica: Vol. 29 No. 1 (2009)
- Harry Pachajoa, Arelis Barragán, Javier Torres, Carolina Isaza, Pentalogy of Cantrell: report of a case with consanguineous parents , Biomedica: Vol. 30 No. 4 (2010)
- Cristian Vallejo, Yolanda Cifuentes, Characterization and six-month follow-up on a cohort of newborns with congenital syphilis , Biomedica: Vol. 36 No. 1 (2016)
- Mary A. García, Luisa Imbachí, Paula M. Hurtado, Gloria Gracia, Ignacio Zarante, Ultrasound detection of congenital anomalies in 76,155 births in the cities of Bogotá and Cali, 2011-2012 , Biomedica: Vol. 34 No. 3 (2014)
- Sandra Patricia Misnaza, Claudia Patricia Roncancio, Isabel Cristina Peña, Franklin Edwin Prieto, Geographic distribution of perinatal mortality due to congenital malformations in Colombia, 1999-2008: An analysis of vital statistics data , Biomedica: Vol. 36 No. 3 (2016)
- María Luz Gunturiz, Liliana Cortés, Ester Liliana Cuevas, Pablo Chaparro, Martha Lucía Ospina, Congenital cerebral toxoplasmosis, Zika and chikungunya virus infections: a case report , Biomedica: Vol. 38 No. 2 (2018)
- Gloria Liliana Porras-Hurtado, Olga Mercedes León-Castañeda, Jaime Molano-Hurtado, Sandra Lorena Quiceno, Harry Pachajoa, Juan José Montoya, Prevalence of birth defects in Risaralda, 2010-2013 , Biomedica: Vol. 36 No. 4 (2016)
- Stefano Tassinari, Samuel Martínez-Vernaza, Nicole Erazo-Morera, María Camila Pinzón-Arciniegas, Gloria Gracia, Ignacio Zarante, Epidemiology of congenital heart diseases in Bogotá, Colombia, from 2001 to 2014: Improved surveillance or increased prevalence? , Biomedica: Vol. 38 No. Sup.1 (2018): Suplemento 1, Enfermedades crónicas
- Estephania Candelo, Gabriela Caicedo, Max Feinstein, Harry Pachajoa, Microcephaly in Colombia before the Zika outbreak: A systematic literature review , Biomedica: Vol. 38 No. Sup. 2 (2018): Suplemento 2, Medicina tropical
- Luisa F. Imbachi, Lina M. Ibañez , Paula Hurtado-Villa, Health status and barriers in health care for children with birth defects born between 2011 and 2017 in two institutions in Cali , Biomedica: Vol. 40 No. 1 (2020)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























