Praziquantel-encapsulated niosomes against Schistosoma mansoni with reduced sensitivity to praziquantel
Abstract
Introduction: Praziquantel (PZQ) is the only commercially available drug for schistosomiasis. The current shortage of alternative effective drugs and the lack of successful preventive measures enhance its value. The increase in the prevalence of PZQ resistance under sustained drug pressure is, therefore, an upcoming issue.
Objective: To overcome the tolerance to PZQ using nanotechnology after laboratory induction of a Schistosoma mansoni isolate with reduced sensitivity to the drug during the intramolluscan phase.
Materials and methods: Shedding snails were treated with PZQ doses of 200 mg/kg twice/ week followed by an interval of one week and then repeated twice in the same manner. The success of inducing reduced sensitivity was confirmed in vitro via the reduction of cercarial response to PZQ regarding their swimming activity and death percentage at different examination times.
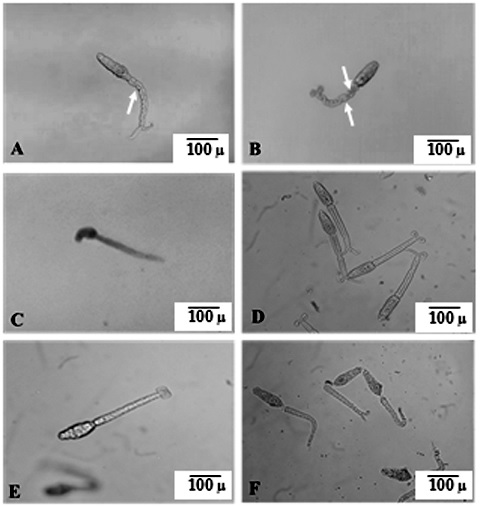
Results: Oral treatment with a single PZQ dose of 500 mg/kg in mice infected with cercariae with reduced sensitivity to PZQ revealed a non-significant reduction (35.1%) of total worm burden compared to non-treated control mice. Orally inoculated PZQ-encapsulated niosomes against S. mansoni with reduced sensitivity to PZQ successfully regained the pathogen’s sensitivity to PZQ as evidenced by measuring different parameters in comparison to the non-treated infected animals with parasites with reduced sensitivity to PZQ. The mean total worm load was 1.33 ± 0.52 with a statistically significant reduction of 94.09% and complete eradication of male worms. We obtained a remarkable increase in the percentage reduction of tissue egg counts in the liver and intestine (97.68% and 98.56%, respectively) associated with a massive increase in dead eggs and the complete absence of immature stages.
Conclusion: PZQ-encapsulated niosomes restored the drug sensitivity against laboratory-induced S. mansoni adult worms with reduced sensitivity to PZQ.
Downloads
References
Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, Boussinesq M, et al. The global burden of disease study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e3265. https://doi.org/10.1371/journal.pntd.0003265
Xu J, Yu Q, Tchuenté LT, Bergquist R, Sacko M, Utzinger J, et al. Enhancing collaboration between China and African countries for schistosomiasis control. Lancet Infect Dis. 2016;16:376-83. https://doi.org/10.1016/S1473-3099(15)00360-6
Abou-El-Naga IF. Towards elimination of schistosomiasis after 5000 years of endemicity in Egypt. Acta Trop. 2018;181:112-21. https://doi.org/10.1016/j.actatropica.2018.02.005
Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, et al. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932-95. https://doi.org/10.4269/ajtmh.1999.60.932
Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Wynn NB, et al. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl Trop Dis. 2009;3:e504. https://doi.org/10.1371/journal.pntd.0000504
Greenberg RM. New approaches for understanding mechanisms of drug resistance in schistosomes, Parasitology. 2013;140:1534-46. https://doi.org/10.1017/S0031182013000231
Kovač J, Vargas M, Keiser J. In vitro and in vivo activity of R- and S- praziquantel enantiomers and the main human metabolite trans-4-hydroxy-praziquantel against Schistosoma haematobium. Parasit Vectors. 2017;10:365. https://doi.org/10.1186/s13071-017-2293-3
Fallon PG, Sturrock RF, Niang AC, Doenhoff MJ. Short report: Diminished susceptibility to praziquantel in a Senegal isolate of Schistosoma mansoni. Am J Trop Med Hyg. 1995;53:61-2.
Pinto-Almeida A, Mendes T, Armada A, Belo S, Carrilho E, Viveiros M, Afonso A. The role of efflux pumps in Schistosoma mansoni praziquantel resistant phenotype. PLoS ONE. 2015;10:e0140147. https://doi.org/10.1371/journal.pone.0140147
Couto FF, Coelho PM, Araújo N, Kusel JR, Katz N, Jannotti-Passos LK, et al. Schistosoma mansoni: A method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem Inst Oswaldo Cruz. 2011;106:153-7. https://doi.org/10.1590/s0074-02762011000200006
Lotfy WM, Hishmat MG, El Nashar AS, Abu El Einin HM. Evaluation of a method for induction of praziquantel resistance in Schistosoma mansoni. Pharm Biol. 2015;53:1214-9. https://doi.org/10.3109/13880209.2014.970289
Kasinathan RS, Greenberg RM. Pharmacology and potential physiological significance of schistosome multidrug resistance transporters. Exp Parasitol. 2012;132:2-6. https://doi.org/10.1016/j.exppara.2011.03.004
Abou-El-Naga IF, El Kerdany ED, Mady RF, Shalaby TI, Zaytoun EM. The effect of lopinavir/ritonavir and lopinavir/ritonavir loaded PLGA nanoparticles on experimental toxoplasmosis. Parasitol Int. 2017;66:735-47. https://doi.org/10.1016/j.parint.2017.08.007
Yang L,Geng Y, Li H, Zhang Y, You J, Chang Y. Enhancement the oral bioavailability of praziquantel by incorporation into solid lipid nanoparticles. Pharmazie. 2009;64:86-9. https://doi.org/10.1691/ph.2009.8140
15. Frezza TF, Gremião MPD, Zanotti-Magalhães EM, Magalhães LA,Souza ALR, Allegretti SM. Liposomal-praziquantel: Efficacy against Schistosoma mansoni in a preclinical assay. Acta Trop. 2013;128:70-5. https://doi.org/10.1016/j.actatropica.2013.06.011
Kolenyak-Santos F, Garnero C, de Oliveira RN, de Souza AL, Chorilli M, Allegretti SM, et al. Nanostructured lipid carriers as a strategy to improve the in vitro Schistosomiasis activity of praziquantel. J Nanosci Nanotechnol. 2015;15:761-72. https://doi.org/10.1166/jnn.2015.9186
Gupta D, Singh A, Khan AU. Nanoparticles as efflux pump and biofilm inhibitor to rejuvenate bactericidal effect of conventional antibiotics. Nanoscale Res Lett. 2017;12:454. https://doi.org/10.1186/s11671-017-2222-6
Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int J Nanomedicine. 2017;12:1227-49. https://doi.org/10.2147/IJN.S121956
Zhang D, Kong YY, Sun JH, Huo SJ, Zhou M, Gui YL, et al. Co-delivery nanoparticles with characteristics of intracellular precision release drugs for overcoming multidrug resistance. Int J Nanomedicine. 2017;12:2081-108. https://doi.org/10.2147/IJN.S128790
Moles E, Urbán P, Jiménez-Díaz MB, Viera-Morilla S, Angulo-Barturen I, Busquets MA, et al. Immunoliposome-mediated drug delivery to Plasmodium-infected and non-infected red blood cells as a dual therapeutic/prophylactic antimalarial strategy. J Control Release. 2015;210:217-29. https://doi.org/10.1016/j.jconrel.2015.05.284
Ahmad K, Rabbani G, Baig MH, lim JH, Khan ME, Lee EJ, et al. Nanoparticle-based drugs: An armament for effective anti-cancer therapy. Curr Drug Metab. 2017;19:839-46. https://doi.org/10.2174/1389200218666170823115647
Sankar V, Ruckmani K, Durga S, Jailani S. Proniosomes as drug carriers. Pak J Pharm Sci. 2010;23:103-7.
Jadon PS, Gajbhiye V, Jadon RS, Gajbhiye KR, Ganesh N. Enhanced Oral Bioavailability of Griseofulvin via Niosomes. AAPS PharmSciTech. 2009;10:1186-92. https://doi.org/10.1208/s12249-009-9325-z
Khan R, Irchhaiya R. Niosomes: A potential tool for novel drug delivery. J Pharmaceut Investig. 2016;46:195-204.
Shingitha KP. A review: Niosomes a novel tool for drug delivery. Int J Appl Biol Pharm. 2015;6:3017-26.
Kulsirirat T, Rukthong P, Dechwongya P, Sathirakul K. The potential of non-ionic surfactant against P-glycoprotein efflux transporters for drug development system. J Bioequiv Bioavail. 2017;9:528-9.
Yolles TK, Moore PV, De-Ginsti DL, Ripson CA, Meleney HE. A technique for the perfusion of laboratory animals for the recovery of schistosomes. J Parasitol. 1947;33:419-26. 28. Abou-El-Naga IF, Sadaka HA, Amer EI, Diab IH, S.I. Khedr SI. Impact of the age of Biomphalaria alexandrina snails on Schistosoma mansoni transmission: modulation of the genetic outcome and the internal defense system of the snail. Mem Inst Oswaldo Cruz. 2015;110:585-95. https://doi.org/10.1590/0074-02760150016
Lewis FA, Stirewalt MA, Souza CP, Gazzinelli G. Large-scale laboratory maintenance of Schistosoma mansoni, with observations on three schistosome/snail host combinations. J Parasitol. 1986;72:813-29.
Abou-El-Naga IF, Amer EI, Boulos LM, El-Faham MH, Abou Seada NM, Younis SS. Biological and proteomic studies of Schistosoma mansoni with decreased sensitivity to
praziquantel. Comp Immunol Microbiol Infect Dis. 2019;66:101341. https://doi.org/10.1016/j.cimid.2019.101341
Liang YS, Bruce JL, Boyd DA. Laboratory cultivation of schistosome vector snails and maintenance of schistosome life cycles. Proc First Sino-Am Symp. 1987;1:34-48.
Lotfy WM, Zaki A, El-Sayed SM. A study on a cercarial assay for detection of praziquantel-resistance in Alexandria (Egypt). Parasitologists United Journal. 2009;2:25-32.
Gonnert R, Andrews P. Praziquental, a new broad spectrum antischistosomal agent. Z Parasitol. 1977;52:129-50.
Bayindir ZS, Yuksel N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci. 2010;99:2049-60. https://doi.org/10.1002/jps.21944
Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: A controlled and novel drug delivery system. Bio Pharmaceut Bull. 2011;34:945-53. https://doi.org/10.1248/bpb.34.945
Lawrence MJ. Formation, characterization and stability of nonionic surfactant vesicles. STP Pharma Sci. 1996;6:49-60.
Kiwada H, Niimura H, Kato Y. Tissue distribution and pharmacokinetic evaluation of the targeting efficiency of synthetic alkyl glycoside vesicles. Chem Pharm Bull. 1985;33:2475-82.
Tian M, Gao Y, Liu Y, Liao Y, Xu R, Hedin NE, et al. Bis-GMA/TEGDMA Dental composites reinforced with electrospun nylon 6 nanocomposite nanofibers containing highly aligned fibrillar silicate single crystals. Polymer (Guildf). 2007;48:2720-8. https://doi.org/10.1016/j.polymer.2007.03.032
Smithers SR, Terry RT. Infection of laboratory hosts with cercaria of Schistosoma mansoni and the recovery of adult worms. Parasitology. 1965;55:695-700.
Cheever AW. Conditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissues. Bull World Health Organ. 1968;39:328-31.
Pellegrino J, Faria J. The oogram method for the screening of drugs in schistosomiasis mansoni. Am J Trop Med Hyg. 1965;14:363-9. https://doi.org/10.4269/ajtmh.1965.14.363
Dmitrienko A, Hsu JC. Multiple testing in clinical trials. In: Kotz S, Balakrishnan N, Read CB, Vidakovic B, editors. Encyclopedia of statistical sciences. 2nd edition. Hoboken (NJ): John Wiley & Sons; 2006.
de Freitas Tallarico L, Miyasato PA, Nakano E. Rearing and maintenance of Biomphalaria glabrata (Say, 1818): Adults and embryos under laboratory conditions. Ann Aquacult Res. 2016;3:1013.
Mattos AC, Kusel JR, Pimenta PF, Coelho PM. Activity of praziquantel on in vitro transformed Schistosoma mansoni sporocysts. Mem Inst Oswaldo Cruz. 2006;101:283-7. https://doi.org/10.1590/s0074-02762006000900044
Liang YS, Coles GC, Doenhoff MJ, Southgate VR. In vitro responses of praziquantel-resistant and susceptible Schistosoma mansoni to praziquantel. Int J Parasitol. 2001;31:1227-35. https://doi:10.1166/jnn.2015.9186
Coles GC, Kinoti GK. Defining resistance in Schistosoma. Parasitol Today. 1997;13:157-8. https://doi.org/10.1016/s0169-4758(97)89815-8
Abou-El-Naga IF. Heat shock protein 70 (Hsp70) in Schistosoma mansoni and its role in decreased adult worm sensitivity to praziquantel. Parasitology. 2020;147:634-42. https://doi.org/10.1017/S0031182020000347
Abou-El-Naga IF. Schistosoma mansoni sarco/endoplasmic reticulum Ca2+ ATPases (SERCA): Role in reduced sensitivity to praziquantel. J Bioenerg Biomembr. 2020;52:397-408. https://doi.org/10.1007/s10863-020-09843-7
Hanallah S, El-Lakkany NM, Mahmoud S, Mousa M, Botros S. Altered immunoglobulin isotype profile and anti-immature worm surface immunoglobulins in mice harboring a praziquantel-resistant Schistosoma mansoni isolate. APMIS. 2003;111:1125-32. https://doi.org/10.1111/j.1600-0463.2003.apm1111208.x
Botros SS, El-Din SH, El-Lakkany NM, Sabra AN, Ebeid FA. Drug-metabolizing enzymes and praziquantel bioavailability in mice harboring Schistosoma mansoni isolates of different drug susceptibilities. J Parasitol. 2006;92:1344-9. https://doi.org/10.1645/GE-865R.1
Sheweita SA, Mangoura SA, El-Shemi AG. Different levels of Schistosoma mansoni infection induced changes in drug-metabolizing enzymes. J Helminthol. 1998;72:71-7. https://doi.org/10.1017/S0022149X00001012
Badawi AF, Mostafa MH. Possible mechanisms of alteration in the capacity of carcinogen-metabolizing enzymes during schistosomiasis. J Int Med Res. 1993;21:281-305. https://doi.org/10.1177/030006059302100601
Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid-Based Complement Alternat Med. 2015;246012. https://doi.org/10.1155/2015/246012
Lawrence MJ. Sorbitan esters (sorbitan fatty acid esters). In: Rowe RC, Sheskey PL, Weller PL, editors. Handbook of pharmaceutical excipients, London, UK: Pharmaceutical Press; 2003.
World Health Organization & Joint FAO/WHO Expert committee on food additives. Evaluation of certain food additives: Seventy-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneve: WHO; 2015. p. 124.
AL-Noshokaty TM, Aly I, Abo-Elmatty DM, Mesbah NM, Shehata AS, Etewa S. Evaluation of solid lipid nanoparticles loaded with praziquantel for treatment of Schistosoma mansoni infected rats. Res J Parasitol. 2020;15:38-46. https://doi.org/10.3923/jp.2020.38.46
Bruni N, Stella B, Giraudo L, Della Pepa C, Gastaldi D, Dosio F. Nanostructured delivery systems with improved leishmanicidal activity: A critical review. Int J Nanomedicine. 2017;12:5289-311. https://doi.org/10.2147/IJN.S140363
El-Lakkany N, Seif El-Din SH, Heikal L. Bioavailability and in vivo efficacy of a praziquantelpolyvinylpyrrolidone solid dispersion in Schistosoma mansoni-infected mice. Eur J Drug Metab Pharmacokinet. 2012;37:289-99. https://doi.org/10.1007/s13318-012-0089-6
Kasinathan RS, Sharma LK, Cunningham C, Webb TR, Greenberg RM. Inhibition or knockdown of ABC transporters enhances susceptibility of adult and juvenile schistosomes to praziquantel. PLoS Negl Trop Dis. 2014;8:e2865. https://doi.org/10.1371/journal.pntd.0002865
Zhao L, Zhao Y, Schwarz B, Mysliwietz J, Hartig R, Camaj P, et al. Verapamil inhibits tumor progression of chemotherapy-resistant pancreatic cancer side population cells. Int J Oncol. 2016;49:99-110. https://doi.org/10.3892/ijo.2016.3512
Some similar items:
- Leidy González, Jorge Alberto Cortés, Systematic review of antimicrobial resistance in Enterobacteriaceae isolates from Colombian hospitals , Biomedica: Vol. 34 No. 2 (2014)
- María Consuelo Garzón, Dailyn Yorledy Angée, Claudia Llerena, Dora Leticia Orjuela, Jorge Ernesto Victoria, Surveillance of Mycobacterium tuberculosis resistance to antituberculosis drugs , Biomedica: Vol. 28 No. 3 (2008)
- Silvia Blair, Eliana Arango, Jaime Carmona Fonseca, In vitro susceptibility of Colombian Plasmodium falciparum isolates to different antimalarial drugs , Biomedica: Vol. 28 No. 2 (2008)
- César A. Arias, Marylin Hidalgo, Jinnethe Reyes, Ana María Cárdenas, Lorena Díaz, Sandra Ríncon, Natasha Vanegas, Paula Lucía Díaz, Elizabeth Castañeda, Resistance profiles to fluoroquinolones in clinical isolates of Gram positive cocci , Biomedica: Vol. 28 No. 2 (2008)
- Ana María Perilla, Camilo González, Sandra Liliana Valderrama, Natasha Vanegas, Bibiana Chavarro, Luis Carlos Triana, José Roberto Támara, Carlos Arturo Álvarez, Necrotizing pneumonia by community-acquired, methicillin-resistant Staphylococcus aureus in Colombia , Biomedica: Vol. 29 No. 4 (2009)
- Claudia Llerena, Santiago Elías Fadul, María Consuelo Garzón, Graciela Mejía, Dora Leticia Orjuela, Luz Mary García, Hilda Beatriz Álvarez, Fernando Javier Ruiz, Drug-resistant Mycobacterium tuberculosis in children under 15 years , Biomedica: Vol. 30 No. 3 (2010)
- David Felipe Briceño, Adriana Correa, Carlos Valencia, Julián Andrés Torres, Robinson Pacheco, María Camila Montealegre, Diego Ospina, María Virginia Villegas, Grupo de Resistencia Bacteriana Nosocomial, Antimicrobial resistance of Gram negative bacilli isolated from terciary-care hospitals in Colombia , Biomedica: Vol. 30 No. 3 (2010)
- Marcela López, Claudia Álvarez, Utility of nitrate reductase assay for detection of multidrug-resistant Mycobacterium tuberculosis in a low resource setting , Biomedica: Vol. 31 No. 2 (2011)
- Ingrid Yamile Pulido, José Ramón Mantilla, Emilia María Valenzuela, María Teresa Reguero, Elsa Beatriz González, Distribution of extended spectrum β-lactamases-codifying genes in Klebsiella pneumoniae isolates from hospitals of Bogota, D.C., Colombia , Biomedica: Vol. 31 No. 1 (2011)
- Carmelo José Espinosa, Jorge Alberto Cortés, Juan Sebastián Castillo, Aura Lucía Leal, Systematic review of antimicrobial resistance among Gram positive cocci in hospitals in Colombia , Biomedica: Vol. 31 No. 1 (2011)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























