Efficacy and safety of four COVID-19 vaccines in preventing SARS-CoV-2 infection: A rapid review
Abstract
Introduction: Since the emergence of the SARS-CoV-2, there have been efforts to develop vaccines to control the COVID-19 pandemic.
Objective: The present study assessed the efficacy and safety of the BNT162b2, mRNA-1273, ChAdOx1/AZD1222 and Gam-COVID-Vac rAd26-S/rAd5-S vaccines against the
SARS-CoV-2.
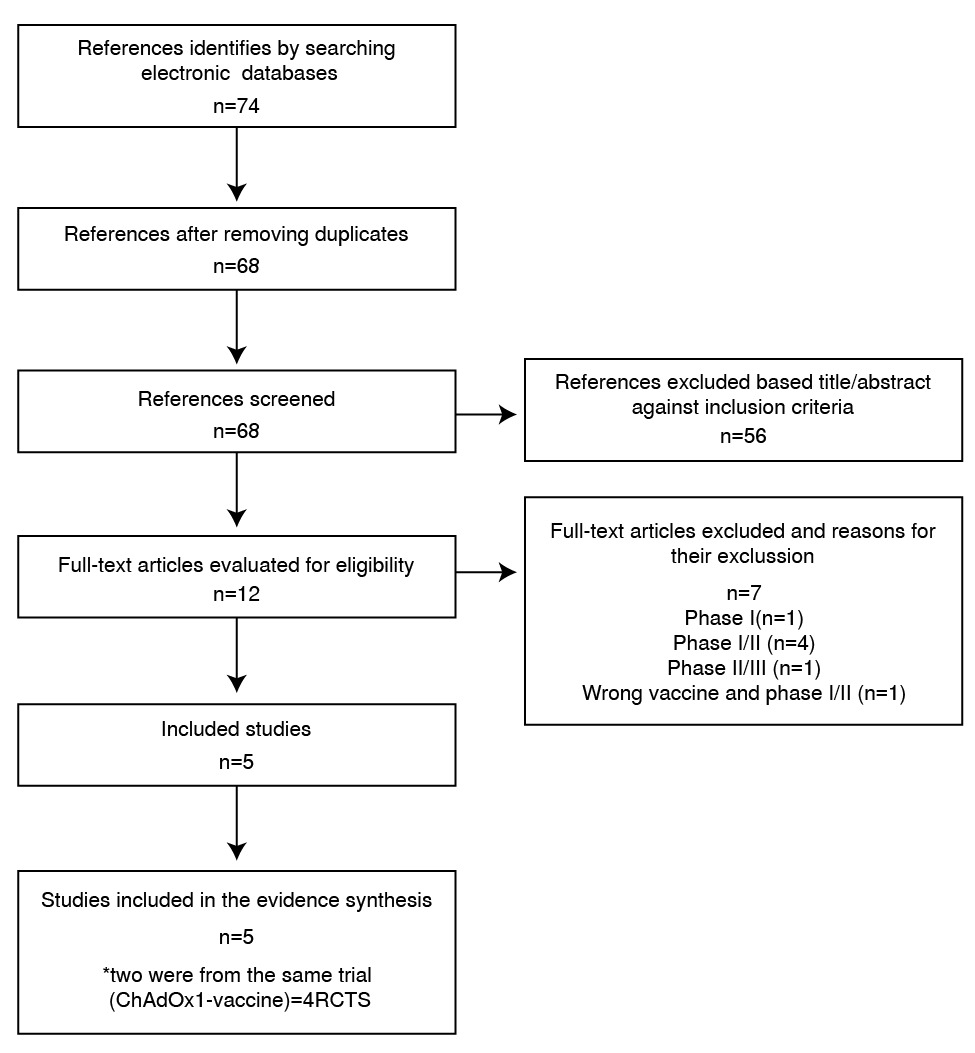
Materials and methods: We searched PubMed/MEDLINE, Google Scholar, Cochrane, and the WHO International Clinical Trials Registry Platform on March 15, 2021. The search
terms used were: “vaccine” OR “vaccination” AND “covid19” OR “coronavirus” OR “sarscov2” AND “bnt162b2” OR “chadox1-S” OR “azd1222” OR “sputnik” OR “Gam-COVID-Vac” OR
“mrna” OR “mRNA-1273” . We measured the risk of bias of the studies and the quality of the evidence using GRADE profiles. A qualitative and quantitative analysis of the results of clinical trials is presented.
Results: Of the 74 identified studies, 4 were finally included in this review. The efficacies of the BNT162b2, mRNA-1273, ChAdOx1/AZD1222 and Gam-COVID-VacrAd26-S/rAd5-S vaccines against symptomatic COVID-19 were 95,0% (CI95% 90,3-97,6), 94,1% (CI95% 89,3-96,8), 66,7% (CI95% 57,4-74,0), and 91,1% (CI95% 83,8-95,1), respectively. There was moderate certainty of the evidence due to serious indirectness, when we measured the risk of bias of the studies and the quality of the evidence using GRADE profile. The safety profiles were acceptable, and data on serious adverse events (summary RR=0,93; CI95% 0,77-1,12; p=0,16) and deaths from all causes (summary RR=0,70; CI95% 0,33-1,50; p=0,90) showed no significant differences.
Conclusion: The results of this review support the level of evidence for the efficacy and safety of the COVID-19 vaccines analysed.
Downloads
References
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265-69. https://doi.org/10.1038/s41586-020-2008-3
Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;4:536-44. https://doi.org/10.1038/s41564-020-0695-z
World Health Organization. Coronavirus disease (COVID-19) pandemic, dashboard. Accessed: June 21, 2021. Available at: https://covid19.who.int/
Schuchat A. Human vaccines and their importance to public health. Procedia in Vaccinology. 2011;5:120-6. https://doi.org/10.1016/j.provac.2011.10.008
ClinicalTrials.gov. COVID, SARS-CoV-2, Vaccination. Accessed: June 21, 2021. Available at: https://clinicaltrials.gov/ct2/results?cond=Covid19&term=vaccines&cntry=&state=&city=&dist=
World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. Accessed: June 21, 2021. Available at: https://www.who.int/publications/m/item/draftlandscape-of-covid-19-candidate-vaccines
Haghpanah F, Lin G, Levin SA, Klein E. Analysis of the potential impact of durability, timing, and transmission blocking of COVID-19 vaccine on morbidity and mortality. EClinicalMedicine. 2021;35:1-13. https://doi.org/10.1016/j.eclinm.2021.100863
Food and Drug Administration. Moderna COVID-19 Vaccine, Emergency Use Authorization (EUA) of the Moderna covid-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Accessed: April 21, 2021. Available at: https://www.fda.gov/emergency-preparedness-andresponse/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
Food and Drug Administration. Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. Accessed: April 21, 2021. Available at: https://www.fda.gov/emergency-preparedness-andresponse/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine
AztraZeneca. AstraZeneca’s COVID-19 vaccine authorised for emergency supply in the UK. Accessed: April 21, 2021. Available at: https://www.astrazeneca.com/media-centre/pressreleases/2020/astrazenecas-covid-19-vaccine-authorised-in-uk.html
World Health Organization. Pfizer/BioNTech COMIRNATY®, COVID-19 vaccine. Accessed: April 22, 2021. Available at: https://www.who.int/publications/m/item/comirnaty-covid-19-mrna-vaccine
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928
Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126-35. https://doi.org/10.1016/j.jclinepi.2019.10.014
Langan D, Higgins JPT, Simmonds M. Comparative performance of heterogeneity variance estimators in meta-analysis: A review of simulation studies. Res Synth Methods. 2017;2:181-98. https://doi.org/10.1002/jrsm.1198
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;11:1539-58. https://doi.org/10.1002/sim.1186
Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;25:2439-50. https://doi.org/10.1056/NEJMoa2027906
Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) Vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27:270-8. https://doi.org/10.1038/s41591-020-01194-5
Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;7830:589-93. https://doi.org/10.1038/s41586-020-2639-4
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;10249:467-78. https://doi.org/10.1016/S0140-6736(20)31604-4
Barrett JR, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;2:279-88. https://doi.org/10.1038/s41591-020-01179-4
Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;10267:1979-93. https://doi.org/10.1016/S0140-6736(20)32466-1
Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1-2 Trial of a SARSCoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;24:2320-32. https://doi.org/10.1056/NEJMoa2026920
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;27:2603-15. https://doi.org/10.1056/NEJMoa2034577
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-16. https://doi.org/10.1056/NEJMoa2035389
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;10269:99-111. https://doi.org/10.1016/S0140-6736(20)32661-1
Voysey M, Costa-Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Singledose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet. 2021;10277:881-91. https://doi.org/10.1016/S0140-6736(21)00432-3
Logunov DY, Dolzhikova I V, Shcheblyakov D V, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;10275:671-81. https://doi.org/10.1016/S0140-6736(21)00234-8
Centers for Disease Control and Prevention. Science Brief: Background rationale and evidence for public health recommendations for fully vaccinated people. Accessed: April 22, 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fullyvaccinated-people.html
World Health Organization. Target product profiles for COVID-19 vaccines. Accessed: May 10, 2021. Available at: https://www.who.int/publications/m/item/who-target-product-profiles-forcovid-19-vaccines
World Health Organization. WHO issues it’s first emergency use validation for a COVID-19 vaccine and emphasizes need for equitable global access. Accessed: April 10, 2021. Available at: https://www.who.int/news/item/31-12-2020-who-issues-its-first-emergency-usevalidation-for-a-covid-19-vaccine-and-emphasizes-need-for-equitable-global-access
Sinopharm. Chinese Covid-19 vaccine efficacy better than expected. Accessed: April 10, 2021. Available at: https://www.precisionvaccinations.com/vaccines/sinopharm-covid-19-vaccine-bbibp-corv
Mallapaty S. China COVID vaccine reports mixed results — what does that mean for the pandemic? Accessed: April 10, 2021. Available at: https://www.nature.com/articles/d41586-021-00094-z
Centers for Disease Control and Prevention. Johnson & Johnson’s Janssen COVID-19 vaccine overview and safety. Accessed: May 10, 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html
Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine. Accessed: May 10, 2021. Available at: https://www.fda.gov/news-events/pressannouncements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
Grant MJ, Booth A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;2:91-108. https://doi.org/10.1111/j.1471-1842.2009.00848.x
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13:224. https://doi.org/10.1186/s12916-015-0465-6
Alberta Health Services. COVID-19 Scientific Advisory Group evidence summary and recommendations. Accessed: May 10, 2021. Available at: https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-sag-post-vaccine-transmission-rapid-review.pdf
Centers for Disease Control and Prevention. CDC issues first set of guidelines on how fully vaccinated people can visit safely with others. Accessed: May 10, 2021. Available at: https://www.cdc.gov/media/releases/2021/p0308-vaccinated-guidelines.html
Some similar items:
- José Moreno-Montoya, Epidemiology of self-care beyond the individual and the sanitary spheres , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Ana María Vásquez, Felipe Sanín, Luis Gonzalo Álvarez, Alberto Tobón, Alexandra Ríos, Silvia Blair, Therapeutic efficacy of a regimen of artesunate-mefloquine-primaquine treatment for Plasmodium falciparum malaria and treatment effects on gametocytic development , Biomedica: Vol. 29 No. 2 (2009)
- Ricardo Sánchez, Gerardo Téllez, Luis Eduardo Jaramillo, Age of onset symptoms and gender in schizophrenic spectrum disorders , Biomedica: Vol. 32 No. 2 (2012)
- Luis F. García, Luis F. Barrera, Perspectives for new anti-tuberculous vaccines in the post-genomic era. , Biomedica: Vol. 24 (2004): Suplemento 1
- Mónica Alejandra Bernal-Vargas, Jorge Alberto Cortés, Ricardo Sánchez, Cross-cultural adaptation of the community-acquired pneumonia score questionnaire in patients with mild-to-moderate pneumonia in Colombia , Biomedica: Vol. 37 No. 1 (2017)
- Paola Andrea Palacios, Carolina Duarte, Olga Sanabria, Jaime Moreno, Molecular characterization of non-vaccine Streptococcus pneumoniae serotypes 11A, 15 B/C and 23A recovered from invasive isolates in Colombia , Biomedica: Vol. 37 No. 3 (2017)
- Carolina Wiesner, Cancer research in the SARS-CoV pandemia , Biomedica: Vol. 40 No. 2 (2020)
- Alfredo G. Torres, Vaccines against SARS-CoV-2: Are they a reality for Latin America? , Biomedica: Vol. 40 No. 3 (2020)
- Zulma M. Cucunubá, Latin American scientific research prorities for COVID-19 prevention and control , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Luis Alberto Gómez, Editorial note about SARS-CoV-2 in the pandemic era , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
Copyright (c) 2022 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























