Technical and clinical aspects of the histocompatibility crossmatch assay in solid organ transplantation
Abstract
The presence of antibodies directed against human leukocyte antigens (HLA) expressed on donor cells is a significant risk factor for serious clinical complications after transplantation.
The crossmatch assay is one of the most important tests available for the detection of donor-specific antibodies in potential allograft recipients. Early crossmatch methods utilized complement-dependent cytotoxicity, which is useful for detecting the donor-specific anti-HLA antibodies responsible for hyperacute allograft rejection but lacks adequate sensitivity.
Consequently, more sensitive crossmatch methods have been developed, ultimately leading to the flow cytometry crossmatch as the currently preferred methodology.
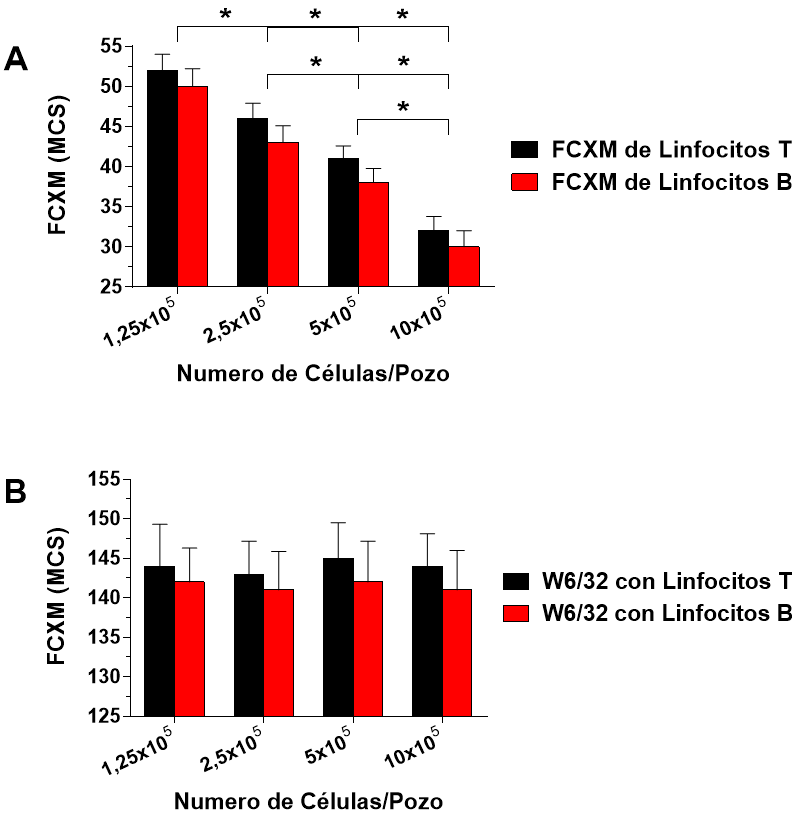
Herein, we review the evolution of the crossmatch assay and the most important factors to consider when performing and interpreting the results of this fundamental assay for ensuring the long-term survival of the transplanted organ.
Downloads
References
Lefaucheur C, Viglietti D, Mangiola M, Loupy A, Zeevi A. From humoral theory to performant risk stratification in kidney transplantation. J Immunol Res. 2017;2017:5201098. https://doi.org/10.1155/2017/5201098
Mehra NK, Baranwal AK. Clinical and immunological relevance of antibodies in solid organ transplantation. Int J Immunogenet. 2016;43:351-68. https://doi.org/10.1111/iji.12294
Valenzuela NM, Reed EF. Antibody-mediated rejection across solid organ transplants: Manifestations, mechanisms, and therapies. J Clin Invest. 2017;127:2492-504.
https://doi.org/10.1172/JCI90597
Loupy A, Lefaucheur C. Antibody-mediated rejection of solid-organ allografts. N Engl J Med. 2018;379:1150-60. https://doi.org/10.1056/NEJMra1802677
Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8:348-57. https://doi.org/10.1038/nrneph.2012.81
Bray RA. Lymphocyte crossmatching by flow cytometry. Methods Mol Biol. 2013;1034:285-96. https://doi.org/10.1007/978-1-62703-493-7_14
Bray RA, Tarsitani C, Gebel HM, Lee JH. Clinical cytometry and progress in HLA antibody detection. Methods Cell Biol. 2011;103:285-310. https://doi.org/10.1016/B978-0-12-385493-3.00012-7
Downing J. The lymphocyte crossmatch by flow cytometry for kidney transplantation. Methods Mol Biol. 2012;882:379-90. https://doi.org/10.1007/978-1-61779-842-9_22
Graff RJ, Buchanan PM, Dzebisashvili N, Schnitzler MA, Tuttle-Newhall J, Xiao H, et al. The clinical importance of flow cytometry crossmatch in the context of CDC crossmatch results. Transplant Proc. 2010;42:3471-4. https://doi.org/10.1016/j.transproceed.2010.06.025
Jaramillo A, Ramon DS. The crossmatch assay in solid organ transplantation. In: Werner J, editor. Introduction to Flow Cytometry. Hauppauge, NY, USA: Nova Science Publishers, Inc., 2019. p. 237-73.
Jaramillo A, Ramón DS, Stoll ST. Technical aspects of crossmatching in transplantation. Clin Lab Med. 2018;38:579-93. https://doi.org/10.1016/j.cll.2018.07.002
Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735-9. https://doi.org/10.1056/NEJM196904032801401
Stiller CR, Sinclair NR, Abrahams S, Ulan RA, Fung M, Wallace AC. Lymphocyte-dependent antibody and renal graft rejection. Lancet. 1975;1:953-4. https://doi.org/10.1016/S0140-6736(75)92010-3
Takasugi M, Sengar DP, Terasaki PI. Microassays in transplantation immunology. Am J Med Technol. 1971;37:470-2.
Tanaka N, Takasugi M, Terasaki PI. Presensitization to transplants detected by cellular immunity tests. Transplantation. 1971;12:514-8. https://doi.org/10.1097/00007890-197112000-00017
Terasaki PI, McClelland JD. Microdroplet assay of human serum cytotoxins. Nature. 1964;204:998-1000. https://doi.org/10.1038/204998b0
Amos DB, Cohen I, Klein WJ Jr. Mechanisms of immunologic enhancement. Transplant Proc. 1970;2:68-75.
Fuller TC, Fuller AA, Golden M, Rodey GE. HLA alloantibodies and the mechanism of the antiglobulin-augmented lymphocytotoxicity procedure. Hum Immunol. 1997;56:94-105. https://doi.org/10.1016/S0198-8859(97)00174-2
Kerman RH, Kimball PM, van Buren CT, Lewis RM, DeVera V, Baghdahsarian V, et al. Improved renal allograft survival for AHG and DTE/AHG crossmatch-negative recipients. Transplant Proc. 1991;23:400-2.
Johnson AH, Rossen RD, Butler WT. Detection of alloantibodies using a sensitive antiglobulin microcytotoxicity test: Identification of low levels of pre-formed antibodies in accelerated allograft rejection. Tissue Antigens. 1972;2:215-26. https://doi.org/10.1111/j.1399-0039.1972.tb00138.x
Kerman RH, Kimball PM, van Buren CT, Lewis RM, DeVera V, Baghdahsarian V, et al. AHG and DTE/AHG procedure identification of crossmatch-appropriate donor-recipient pairings that result in improved graft survival. Transplantation. 1991;51:316-20. https://doi.org/10.1097/00007890-199102000-00008
Kerman RH. The role of crossmatching in organ transplantation. Arch Pathol Lab Med. 1991;115:255-9.
Soltis RD, Hasz D, Morris MJ, Wilson ID. The effect of heat inactivation of serum on aggregation of immunoglobulins. Immunology. 1979;36:37-45.
Delgado JC, Eckels DD. Positive B-cell only flow cytometric crossmatch: Implications for renal transplantation. Exp Mol Pathol. 2008;85:59-63. https://doi.org/10.1016/j.yexmp.2008.03.009
Duquesnoy RJ, Marrari M. Multilaboratory evaluation of serum analysis for HLA antibody and crossmatch reactivity by lymphocytotoxicity methods. Arch Pathol Lab Med. 2003;127:149-56. https://doi.org/10.5858/2003-127-149-MEOSAF
Cross DE, Whittier FC, Weaver P, Foxworth J. A comparison of the antiglobulin versus extended incubation time crossmatch: Results in 223 renal transplants. Transplant Proc. 1977;9:1803-6.
Garovoy MR, Rheinschmidt MA, Bigos M, Perkins H, Colombe B, Feduska N, et al. Flow cytometry analysis: A high technology cross-match technique facilitating transplantation. Transplant Proc. 1983;15:1939-44.
Bray RA. Flow cytometry in the transplant laboratory. Ann N Y Acad Sci. 1993;677:138-51. https://doi.org/10.1111/j.1749-6632.1993.tb38772.x
Bray RA. Flow cytometry crossmatching for solid organ transplantation. Methods Cell Biol. 1994;41:103-19. https://doi.org/10.1016/S0091-679X(08)61712-4
Bray RA, Lebeck LK, Gebel HM. The flow cytometric crossmatch. Dual-color analysis of T cell and B cell reactivities. Transplantation. 1989;48:834-40.
Cresswell P. Regulation of HLA class I and class II antigen expression. Br Med Bull. 1987;43:66-80. https://doi.org/10.1046/j.1365-2567.1997.00258.x
Kao KJ, Riley WJ. Genetic predetermination of quantitative expression of HLA antigens in platelets and mononuclear leukocytes. Hum Immunol. 1993;38:243-50. https://doi.org/10.1016/0198-8859(93)90551-B
Vandiedonck C, Taylor MS, Lockstone HE, Plant K, Taylor JM, Durrant C, et al. Pervasive haplotypic variation in the spliceo-transcriptome of the human major histocompatibility complex. Genome Res. 2011;21:1042-54. https://doi.org/10.1101/gr.116681.110
Good DJ, Zhang A, Kemesky J, Stopczynski N. Previously believed to be nonsensicl crossmatch results, explained by anti-HLA-C antibodies. Hum Immunol. 2017;78:S75. https://doi.org/10.1016/j.humimm.2017.06.090
Inlow J, Steller P, Eltayeb A, Rearick A, Kott M, Adams P, et al. Case study: Positive T-cell flow crossmatch (TFXM)/negative B-cell flow crossmatch (BFXM) can be mediated by a class I antibody. Hum Immunol. 2011;72:S154. https://doi.org/10.1016/j.humimm.2011.07.245
Lucas DP, Vega RM, Jackson AM. Variable expression of HLA-C impacts T versus B cell crossmatch outcomes. Hum Immunol. 2016;77:S2. https://doi.org/10.1016/j.humimm.2016.07.014
Badders JL, Jones JA, Jeresano ME, Schillinger KP, Jackson AM. Variable HLA expression on deceased donor lymphocytes: Not all crossmatches are created equal. Hum Immunol. 2015;76:795-800. https://doi.org/10.1016/j.humimm.2015.09.029
Basham TY, Merigan TC. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983;130:1492-4.
Kuipers HF, Biesta PJ, Groothuis TA, Neefjes JJ, Mommaas AM, van den Elsen PJ. Statins affect cell-surface expression of major histocompatibility complex class II molecules by disrupting cholesterol-containing microdomains. Hum Immunol. 2005;66:653-65. https://doi.org/10.1016/j.humimm.2005.04.004
Le Morvan C, Cogne M, Troutaud D, Charmes JP, Sauvage P, Drouet M. Modification of HLA expression on peripheral lymphocytes and monocytes during aging. Mech Ageing Dev. 1998;105:209-20. https://doi.org/10.1016/S0047-6374(98)00096-7
Viallard JF, Bloch-Michel C, Neau-Cransac M, Taupin JL, Garrigue S, Miossec V, et al. HLA-DR expression on lymphocyte subsets as a marker of disease activity in patients with systemic lupus erythematosus. Clin Exp Immunol. 2001;125:485-91. https://doi.org/10.1046/j.1365-2249.2001.01623.x
Liwski R, Adams G, Peladeau G, Heinstein K. The impact of lymphocyte purity on flow cytometry crossmatch (FCXM) assay. It’s not purely theoretical. Hum Immunol. 2016;77:S110-S111. https://doi.org/10.1016/j.humimm.2016.07.164
Liwski RS, Greenshields AL, Conrad DM, Murphey C, Bray RA, Neumann J, et al. Rapid optimized flow cytometric crossmatch (FCXM) assays: The Halifax and Halifaster protocols. Hum Immunol. 2018;79:28-38. https://doi.org/10.1016/j.humimm.2017.10.020
Barrios K, Lunz J, Labuda B, Magas D, Freedom D, Szewczyk K, et al. Optimized flow cytometry crossmatch with increased sensitivity and specificity. Am J Transplant. 2016;16:S609.
Lobo PI, Spencer CE, Isaacs RB, McCullough C. Hyperacute renal allograft rejection from anti-HLA class 1 antibody to B cells--antibody detection by two color FCXM was possible only after using pronase-digested donor lymphocytes. Transpl Int. 1997;10:69-73. https://doi.org/10.1007/BF02044346
Lobo PI, Spencer CE, Stevenson WC, McCullough C, Pruett TL. The use of pronasedigested human leukocytes to improve specificity of the flow cytometric crossmatch. Transpl Int. 1995;8:472-80. https://doi.org/10.1111/j.1432-2277.1995.tb01558.x
Lobo PI, Winfield JB, Craig A, Westervelt FB Jr. Utility of protease-digested human peripheral blood lymphocytes for the detection of lymphocyte-reactive alloantibodies by indirect immunofluorescence. Transplantation. 1977;23:16-21. https://doi.org/10.1097/00007890-197701000-00003
Vaidya S, Cooper TY, Avandsalehi J, Barnes T, Brooks K, Hymel P, et al. Improved flow cytometric detection of HLA alloantibodies using pronase: Potential implications in renal transplantation. Transplantation. 2001;71:422-8. https://doi.org/10.1097/00007890-200102150-00015
Vaidya S, Cooper TY, Stewart D, Gugliuzza K, Daller J, Bray RA. Pronase improves detection of HLA antibodies in flow crossmatches. Transplant Proc. 2001;33:473-4. https://doi.org/10.1016/S0041-1345(00)02098-4
Hetrick SJ, Schillinger KP, Zachary AA, Jackson AM. Impact of pronase on flow cytometric crossmatch outcome. Hum Immunol. 2011;72:330-6. https://doi.org/10.1016/j.humimm.2011.01.005
Park H, Lim YM, Han BY, Hyun J, Song EY, Park MH. Frequent false-positive reactions in pronase-treated T-cell flow cytometric cross-match tests. Transplant Proc. 2012;44:87-90.
https://doi.org/10.1016/j.transproceed.2011.12.048
Hardt M, Baron T, Groschup MH. A comparative study of immunohistochemical methods for detecting abnormal prion protein with monoclonal and polyclonal antibodies. J Comp Pathol. 2000;122:43-53. https://doi.org/10.1053/jcpa.1999.0343
Lipman NS, Jackson LR, Trudel LJ, Weis-García F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J. 2005;46:258-68. https://doi.org/10.1093/ilar.46.3.258
Kerman RH, van Buren CT, Lewis RM, DeVera V, Baghdahsarian V, Gerolami K, et al. Improved graft survival for flow cytometry and antihuman globulin crossmatch-negative retransplant recipients. Transplantation. 1990;49:52-6. https://doi.org/10.1097/00007890-199001000-00011
Bray RA, Nolen JD, Larsen C, Pearson T, Newell KA, Kokko K, et al. Transplanting the highly sensitized patient: The Emory algorithm. Am J Transplant. 2006;6:2307-15. https://doi.org/10.1111/j.1600-6143.2006.01521.x
Zangwill SD, Ellis TM, Zlotocha J, Jaquiss RD, Tweddell JS, Mussatto KA, et al. The virtual crossmatch--a screening tool for sensitized pediatric heart transplant recipients. Pediatr Transplant. 2006;10:38-41. https://doi.org/10.1111/j.1399-3046.2005.00394.x
Amico P, Hirt-Minkowski P, Honger G, Gurke L, Mihatsch MJ, Steiger J, et al. Risk stratification by the virtual crossmatch: A prospective study in 233 renal transplantations. Transpl Int. 2011;24:560-9. https://doi.org/10.1111/j.1432-2277.2011.01235.x
Amico P, Honger G, Steiger J, Schaub S. Utility of the virtual crossmatch in solid organ transplantation. Curr Opin Organ Transplant. 2009;14:656-61. https://doi.org/10.1097/MOT.0b013e328331c169
Bielmann D, Honger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S. Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant. 2007;7:626-32. https://doi.org/10.1111/j.1600-6143.2007.01667.x
Bingaman AW, Murphey CL, Palma-Vargas J, Wright F. A virtual crossmatch protocol significantly increases access of highly sensitized patients to deceased donor kidney transplantation. Transplantation. 2008;86:1864-8. https://doi.org/10.1097/TP.0b013e318191404c
Claas FHJ, Heidt S. Virtual crossmatching for deceased donor transplantation becomes reality. Kidney Int. 2020;97:657-9. https://doi.org/10.1016/j.kint.2020.01.024
Jaramillo A, Reddy KS, Heilman RL. Using the virtual crossmatch to allow for safer and more efficient kidney transplantation of highly sensitized patients. Transplantation. 2020;104:1121-2. https://doi.org/10.1097/TP.0000000000002925
Johnson CP, Schiller JJ, Zhu YR, Hariharan S, Roza AM, Cronin DC, et al. Renal transplantation with final allocation based on the virtual crossmatch. Am J Transplant. 2016;16:1503-15. https://doi.org/10.1111/ajt.13606
Morris GP, Phelan DL, Jendrisak MD, Mohanakumar T. Virtual crossmatch by identification of donor-specific anti-human leukocyte antigen antibodies by solid-phase immunoassay: A 30-month analysis in living donor kidney transplantation. Hum Immunol. 2010;71:268-73. https://doi.org/10.1016/j.humimm.2010.01.003
Rohan VS, Pilch N, Moussa O, Nadig SN, Dubay D, Baliga PK, et al. Virtual crossmatching in kidney transplantation: The wait is over. J Am Coll Surg. 2020;230:373-9. https://doi.org/10.1016/j.jamcollsurg.2019.12.031
Roll GR, Webber AB, Gae DH, Laszik Z, Tavakol M, Mayen L, et al. A virtual crossmatchbased strategy facilitates sharing of deceased donor kidneys for highly sensitized recipients. Transplantation. 2020;104:1239-45. https://doi.org/10.1097/TP.0000000000002924
Some similar items:
- Adriano Martínez, Ismael Reyes, Niradiz Reyes, Cytotoxicity of the herbicide glyphosate in human peripheral blood mononuclear cells , Biomedica: Vol. 27 No. 4 (2007)
- Ricardo Cardona, Ruth Helena Ramírez, Zulma Reina, Mauricio Fernando Escobar, Edison Morales, Allergy and intolerance to nonsteroidal antinflammatory drugs: successful desensitization in three cases , Biomedica: Vol. 29 No. 2 (2009)
- Vanihamín Domínguez, Itzen Aguiñiga, Leticia Moreno, Beatriz Torres, Edelmiro Santiago-Osorio, Sodium caseinate increases the number of B lymphocytes in mouse , Biomedica: Vol. 37 No. 4 (2017)
- María Teresa Rugeles, Paula A. Velilla, Carlos J. Montoya, Mechanisms of human natural resistance to HIV: A summary of ten years of research in the Colombian population , Biomedica: Vol. 31 No. 2 (2011)
- Juan Carlos Villa-Camacho, Juan Camilo Vargas-Zambrano, John Mario González, Flow cytometry model for the detection of neutralizing antibodies against of IFN-β , Biomedica: Vol. 32 No. 4 (2012)
- Viviana Marcela Rodríguez, Adriana Cuéllar, Lyda Marcela Cuspoca, Carmen Lucía Contreras, Marcela Mercado, Alberto Gómez, Phenotypical determinants of stem cell subpopulations derived from human umbilical cord blood. , Biomedica: Vol. 26 No. 1 (2006)
- Jorge Sánchez, María Nelly Restrepo, José Mopan, Carlos Chinchilla, Ricardo Cardona, Milk and egg allergy: Diagnosis, management and implications for Latin America , Biomedica: Vol. 34 No. 1 (2014)
- Lina Marcela Barrera, León Darío Ortiz, Hugo Grisales, Mauricio Rojas, Mauricio Camargo, Flow cytometry in peripheral blood reticulocytes as a marker of chromosome instability in highgrade glioma patients , Biomedica: Vol. 38 No. 3 (2018)
- Yazmin Rocío Arias, Karime Osorio-Arango, Brayan Bayona, Guadalupe Ercilla, Mauricio Beltrán-Durán, Determination of HLA-A, -B and -DRB1 polymorphism in brain dead organ donors representative of the Colombian general population, 2007-2014 , Biomedica: Vol. 37 No. 2 (2017)
- Liliana Fernández, Mauricio Velásquez, Luz Fernanda Sua, Indira Cujiño, Martha Giraldo, Diego Medina, Mauricio Burbano, Germán Torres, Carlos Munoz-Zuluaga, Leidys Gutiérrez-Martínez, The porcine biomodel in translational medical research: From biomodel to human lung transplantation , Biomedica: Vol. 39 No. 2 (2019)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























