Ziehl-Neelsen stain in the pathology laboratory: Performance and diagnostic aid for mycobacteria in bronchoalveolar lavage
Abstract
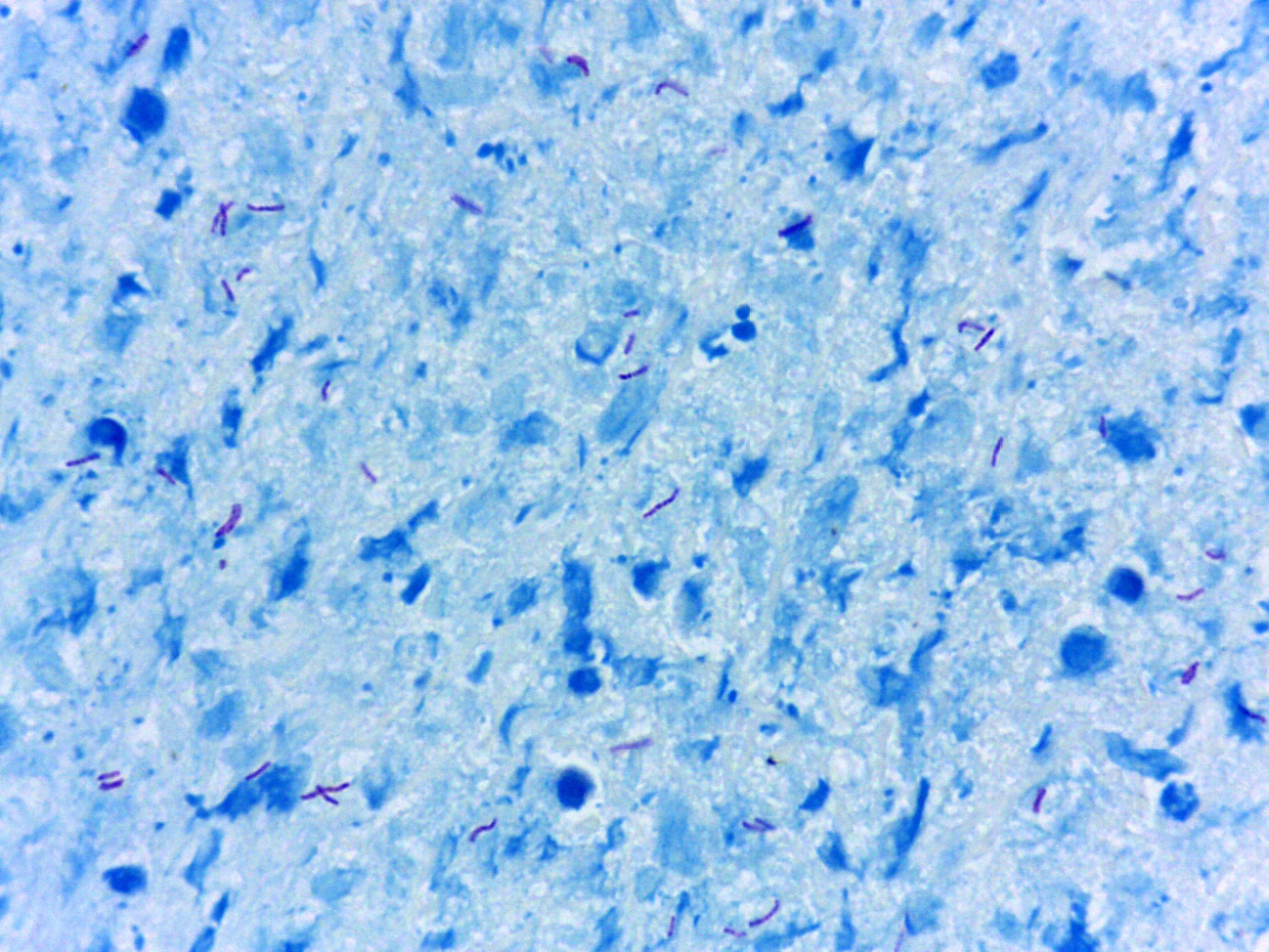
Introduction: With more than 100 years of use, the Ziehl-Neelsen stain is still currently used worldwide.
Objective: To compare the performance of diagnostic tests used to determine mycobacteria in the clinic and pathology laboratory in bronchoalveolar lavage samples.
Materials and methods: We retrospectively reviewed 737 bronchoalveolar lavage samples from 2019 to 2020 in the San Vicente Fundación hospital (Medellín, Colombia) comparing the performance of three tests done in parallel: mycobacteria and resistance PCR, culture, and Ziehl-Neelsen stain.
Results: In total, 93/737 patients were classified as sick due to a positive result in any of the three tests. The culture, PCR, and Ziehl-Neelsen stain had a sensibility of 0.80, 0.76 y 0.51, respectively. However, only 5/75 (6.5%) of the positive cultures had results within the first four weeks and the rest in eight weeks. The PCR test combined with the Ziehl-Neelsen stain improved the sensibility of the PCR test alone from 0.76 a 0.88, a change that was statistically significant (p = 0.022).
Conclusion: At least in bronchoalveolar lavage samples, culture is still the test with better sensibility. The use in parallel of the PCR test and the Ziehl-Neelsen stain improved in a statistically significant manner the performance of the PCR test alone, regardless of the higher turnaround time of the Ziehl-Neelsen stain.
Downloads
References
Khan MK, Islam MN, Ferdous J, Alam MM. An overview on epidemiology of tuberculosis. Mymensingh Med J. 2019;28:259-66.
Pezzella AT. History of pulmonary tuberculosis. Thorac Surg Clin. 2019;29:1-17. https://doi.org/10.1016/j.thorsurg.2018.09.002
Bañuls A-L, Sanou A, van Anh NT, Godreuil S. Mycobacterium tuberculosis: Ecology and evolution of a human bacterium. J Med Microbiol. 2015;64:1261-9. https://doi.org/10.1099/jmm.0.000171
Fadul S. Vigilancia y análisis del riesgo en salud pública, protocolo de vigilancia en salud pública de tuberculosis farmacorresistente. Bogotá, D.C.: Instituto Nacional de Salud; 2019.
Rodríguez-Castillo JG, Llerena C, Argoty-Chamorro L, Guerra J, Couvin D, Rastogi N, et al. Population structure of multidrug-resistant Mycobacterium tuberculosis clinical isolates in Colombia. Tuberculosis (Edinb). 2020;125:102011. https://doi.org/10.1016/j.tube.2020.102011
Santos-Pereira A, Magalhães C, Araújo PMM, S Osório N. Evolutionary genetics of Mycobacterium tuberculosis and HIV-1: “The Tortoise and the Hare.” Microorganisms. 2021;9:147. https://doi.org/10.3390/microorganisms9010147
Gopalaswamy R, Subbian S. Corticosteroids for COVID-19 therapy: potential implications on tuberculosis. Int J Mol Sci. 2021;22:3773. https://doi.org/10.3390/ijms22073773
Anastasopoulou A, Ziogas DC, Samarkos M, Kirkwood JM, Gogas H. Reactivation of tuberculosis in cancer patients following administration of immune checkpoint inhibitors: Current evidence and clinical practice recommendations. J Immunother Cancer. 2019;7:239. https://doi.org/10.1186/s40425-019-0717-7
Naranjo PJ de, Rodríguez G, Rodríguez J, Caldas ML. La coloración de Ziehl-Neelsen en histopatología. Biomédica. 1988;8:84-93. https://doi.org/10.7705/biomedica.v8i3-4.1964
Rincón-Caballero OL, Cano-Romero MA, Aristizábal-Bernal BH. Diagnóstico de tuberculosis pulmonar en lavado broncoalveolar: desempeño de la PCR en comparación con las pruebas microbiológicas de rutina. Med Lab. 2017;23:475-84. https://doi.org/10.36384/01232576.26
Balows A, Hausler WJ Jr, Herrmann KL, Isenberg HD, Shadomy HJ. Manual of Clinical Microbiology. 5th edition. Washington, D.C.: American Society for Microbiology; 1991. p. 1384.
Bodal VK, Bal MS, Bhagat S, Kishan J, Deepika, Brar RK. Fluorescent microscopy and Ziehl-Neelsen staining of bronchoalveolar lavage, bronchial washings, bronchoscopic brushing and post bronchoscopic sputum along with cytological examination in cases of suspected tuberculosis. Indian J Pathol Microbiol. 2015;58:443-7. https://doi.org/10.4103/0377-4929.168849
Elbrolosy AM, El Helbawy RH, Mansour OM, Latif RA. Diagnostic utility of GeneXpert MTB/RIF assay versus conventional methods for diagnosis of pulmonary and extra-pulmonary tuberculosis. BMC Microbiol. 2021;21:144. https://doi.org/10.1186/s12866-021-02210-5
Wasim Yusuf N, Iram S, Zeenat A, Hussain S, Aslam M. Rapid diagnosis of tuberculosis using Xpert MTB/RIF assay - Report from a developing country. Pak J Med Sci. 2015;31:105-10. https://doi.org/10.12669/pjms.311.6970
Zheng L-H, Jia H-Y, Liu X-J, Sun H-S, Du F-J, Pan L-P, et al. Modified cytospin slide microscopy method for rapid diagnosis of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2016;20:456-61. https://doi.org/10.5588/ijtld.15.0733
van Deun A, Hamid Salim A, Aung KJM, Hossain MA, Chambugonj N, Hye MA, et al. Performance of variations of carbolfuchsin staining of sputum smears for AFB under field conditions. Int J Tuberc Lung Dis. 2005;9:1127-33.
Tuberculosis Division - International Union Against Tuberculosis and Lung Disease. Tuberculosis bacteriology--priorities and indications in high prevalence countries: Position of the technical staff of the Tuberculosis Division of the International Union Against Tuberculosis and Lung Disease. Int J Tuberc Lung Dis. 2005;9:355-61.
Karstaedt AS, Jones N, Khoosal M, Crewe-Brown HH. The bacteriology of pulmonary tuberculosis in a population with high human immunodeficiency virus seroprevalence. Int J Tuberc Lung Dis. 1998;2:312-6.
Khan EA, Starke JR. Diagnosis of tuberculosis in children: Increased need for better methods. Emerg Infect Dis. 1995;1:115-23. https://doi.org/10.3201/eid0104.950402
Some similar items:
- Nohora Marcela Mendoza, Marisol García, Liliana Jazmín Cortés, Claudia Vela, Rigoberto Erazo, Pilar Pérez, Olga Lucía Ospina, Javier Darío Burgos, Evaluation of two rapid diagnostic tests, NOW® ICT Malaria Pf/Pv and OptiMAL®, for diagnosis of malaria , Biomedica: Vol. 27 No. 4 (2007)
- Constanza Pardo, Ricardo Cendales, Survival analysis of cervical cancer patients , Biomedica: Vol. 29 No. 3 (2009)
- Raúl Murillo, Ricardo Cendales, Carolina Wiesner, Marion Piñeros, Sandra Tovar, Effectiveness of cytology-based cervical cancer screening in the Colombian health system , Biomedica: Vol. 29 No. 3 (2009)
- Sandra Lorena Girón, Julio César Mateus, Fabián Méndez, Impact of an open waste disposal site on the occurrence of respiratory symptoms and on health care costs of children , Biomedica: Vol. 29 No. 3 (2009)
- José Joaquín Carvajal, Ligia Inés Moncada, Mauricio Humberto Rodríguez, Ligia del Pilar Pérez, Víctor Alberto Olano, Characterization of Aedes albopictus (Skuse, 1894) (Diptera:Culicidae) larval habitats near the Amazon River in Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Andrés Páez, Gloria Rey, Carlos Agudelo, Alvaro Dulce, Edgar Parra, Hernando Díaz-Granados, Damaris Heredia, Luis Polo, Outbreak of urban rabies transmitted by dogs in Santa Marta, northern Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Patricia Escobar, Katherine Paola Luna, Indira Paola Hernández, César Mauricio Rueda, María Magdalena Zorro, Simon L. Croft, In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole , Biomedica: Vol. 29 No. 3 (2009)
- Gustavo Pradilla, Julio César Mantilla, Reynaldo Badillo, Human rabies encephalitis by a vampire bat bite in an urban area of Colombia , Biomedica: Vol. 29 No. 2 (2009)
- Mauricio Beltrán, María Cristina Navas, María Patricia Arbeláez, Jorge Donado, Sergio Jaramillo, Fernando De la Hoz, Cecilia Estrada, Lucía del Pilar Cortés, Amalia de Maldonado, Gloria Rey, Seroprevalence of hepatitis B virus and human immunodeficiency virus infection in a population of multiply-transfused patients in Colombia , Biomedica: Vol. 29 No. 2 (2009)
- Rosa Magdalena Uscátegui, Adriana M. Correa, Jaime Carmona-Fonseca, Changes in retinol, hemoglobin and ferritin concentrations in Colombian children with malaria , Biomedica: Vol. 29 No. 2 (2009)
Copyright (c) 2022 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
Funding data
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























