Detection and expression of SapS, a class C nonspecific acid phosphatase with O-phospho-Ltyrosine- phosphatase activity, in Staphylococcus aureus isolates from patients with chronic osteomyelitis
Abstract
Introduction. The identity of Staphylococcus aureus virulence factors involved in chronic osteomyelitis remains unresolved. SapS is a class C non-specific acid phosphatase and a well-known virulence factor that has been identified in S. aureus strain 154 but in protein extracts from rotting vegetables.
Objective. To identify the SapS gene and characterize the activity of SapS from S. aureus strains: 12 isolates from bone infected samples of patients treated for chronic osteomyelitis and 49 from a database with in silico analysis of complete bacterial genomes.
Materials and methods. The SapS gene was isolated and sequenced from 12 S. aureus clinical isolates and two reference strains; 49 S. aureus strains and 11 coagulase-negative staphylococci were tested using in silico PCR. Culture media semi-purified protein extracts from the clinical strains were assayed for phosphatase activity with p-nitro-phenylphosphate, O-phospho-L-tyrosine, O-phospho-L-serine, and OphosphoL-threonine in conjunction with various phosphatase inhibitors.
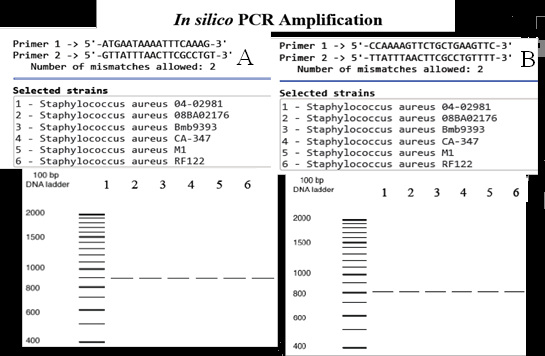
Results. SapS was detected in the clinical and in-silico S. aureus strains, but not in the in silico coagulase-negative staphylococci strains. Sec-type I lipoprotein-type N-terminal signal peptide sequences; secreted proteins, and aspartate bipartite catalytic domains coding sequences were found in the SapS nucleotide and amino acid sequence analysis. SapS dephosphorylated with p-nitro-phenyl-phosphate and ophosphoLtyrosine were selectively resistant to tartrate and fluoride, but sensitive to vanadate and molybdate.
Conclusion. SapS gene was found in the genome of the clinical isolates and the in silico Staphylococcus aureus strains. SapS shares biochemical similarities with known virulent bacterial, such as protein tyrosine phosphatases, suggesting it may be a virulence factor in chronic osteomyelitis.
Downloads
References
Barakat A, Schilling WHK, Sharma S, Guryel E, Freeman R. Chronic osteomyelitis: a review on current concepts and trends in treatment. Orthop Trauma. 2019;33:181-7. https://doi.org/10.1016/j.mporth.2019.03.005
Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369-79. https://doi.org/10.1016/s0140-6736(04)16727-5
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603-61. https://doi.org/10.1128/CMR.00134-14
Ziebandt AK, Kusch H, Degner M, Jaglitz S, Sibbald MJJB, Arends JP, et al. Proteomics uncovers extreme heterogeneity in the Staphylococcus aureus exoproteome due to genomic plasticity and variant gene regulation. Proteomics. 2010;10:1634-44. https://doi.org/10.1002/pmic.200900313
Jin T, Zhu YL, Li J, Shi J, He XQ, Ding J, et al. Staphylococcal protein A, panton-valentin leukocidin and coagulase aggravate the bone loss and bone destruction in osteomyelitis. Cell Physiol Biochem. 2013;32:322-33. https://doi.org/10.1159/000354440
Rossolini GM, Schippa S, Riccio ML, Berlutti F, Macaskie LE, Thaller MC. Bacterial non-specific acid phosphohydrolases: physiology, evolution and use as tools in microbial biotechnology. Cell Mol Life Sci. 1998;54:833-50. https://doi.org/10.1007/s000180050212
Gandhi NU, Chandra SB. A comparative analysis of three classes of bacterial non-specific acid phosphatases and archaeal phosphoesterases: evolutionary perspective. Acta Inform Med. 2012;20:167-73. https://doi.org/10.5455/aim.2012.20.167-73
Du Plessis EM, Theron J, Joubert L, Lotter T, Watson TG. Characterization of a phosphatase secreted by Staphylococcus aureus strain 154, a new member of the bacterial class C family of non-specific acid phosphatases. Syst Appl Microbiol. 2002;25:21-30. https://doi.org/10.1078/0723-2020-00098
Novick RP. Genetic systems in Staphylococci. Meth Enzymol. 1991;204:587636. https://doi.org/10.1016/0076-6879(91)04029-N
Bikandi J, San Millán R, Rementeria A, Garaizar J. In silico analysis of complete bacterial genomes: PCR, AFLP-PCR and endonuclease restriction. Bioinformatics. 2004;20:798-9. https://doi.org/10.1093/bioinformatics/btg491
Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL EBI search and sequence analysis tools. APIs in 2019. Nucleic Acids Res. 2019;47: W636-41. https://doi.org/10.1093/nar/gkz268
Almagro-Armenteros JJ, Tsirigos KD, Sønderby CK, Petersen TN, Winther O, Brunak B, et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol. 2019;37:420-3. https://doi.org/10.1038/s41587-019-0036-z
Shen HB, Chou KC. Gpos-mPLoc: A top-down approach to improve the quality of predicting subcellular localization of gram-positive bacterial proteins. Protein Pept Lett. 2009;16:1478-84. https://doi.org/10.2174/092986609789839322
NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018;46:D8-13. https://doi.org/10.1093/nar/gkx1095
Golovan S, Wang G, Zhang J, Forsberg CW. Characterization and overproduction of the Escherichia coli appA encoded bifunctional enzyme that exhibits both phytase and acid phosphatase activities. Can J Microbiol. 2000;46:59-71. https://doi.org/10.1139/cjm-46-1-59
Hamilton A, Harrington D, Sutcliffe IC. Characterization of acid phosphatase activities in the equine pathogen Streptococcus equi. Syst Appl Microbiol. 2000;23:325-9. https://doi.org/10.1016/S0723-2020(00)80060-0
Zlotnick GW, Gottlieb M. A sensitive staining technique for the detection of phosphohydrolase activities after polyacrylamide gel electrophoresis. Anal Biochem. 1986;153:121-5. https://doi.org/10.1016/0003-2697(86)90069-2
Thaller MC, Schippa S, Rossolini GM. Conserved sequence motifs among bacterial, eukaryotic, and archaeal phosphatases that define a new phosphohydrolase superfamily. Protein Sci. 1998;7:1647-52. https://doi.org/10.1002/pro.5560070722
Kusch H, Engelmann S. Secrets of the secretome in Staphylococcus aureus. Int J Med Microbiol. 2014;304:133–41. https://doi.org/10.1016/j.ijmm.2013.11.005
Bosi E, Monk JM, Aziz RK, Fondi M, Nizet V, Palsson BØ. Comparative genome-scale modelling of Staphylococcus aureus strains identifies strain-specific metabolic capabilities linked to pathogenicity. Proc Natl Acad Sci USA. 2016;113:E38019. https://doi.org/10.1073/pnas.1523199113
Feil EJ, Cooper JE, Grundmann H, Robinson DA, Enright MC, Berendt T, et al. How clonal is Staphylococcus aureus? J Bacteriol. 2003;185:3307-16. https://doi.org/10.1128/jb.185.11.3307-3316.2003
Sibbald MJ, Ziebandt AK, Engelmann S, Hecker M, de Jong A, Harmsen HJ, et al. Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol Mol Biol Rev. 2006;70:755-88. https://doi.org/10.1128/MMBR.00008-06
Caselli A, Paoli P, Santi A, Mugnaioni C, Toti A, Camici G, et al. Low molecular weight protein tyrosine phosphatase: Multifaceted functions of an evolutionarily conserved enzyme. Biochim Biophys Acta. 2016;1864:1339-55. https://doi.org/10.1016/j.bbapap.2016.07.001
Reilly TJ, Chance DL, Calcutt MJ, Tanner JJ, Felts RL, Waller SC, et al. Characterization of a unique class C acid phosphatase from Clostridium perfringens. Appl Environ Microbiol. 2009;75:3745-54. https://doi.org/10.1128/AEM.01599-08
Saleh MT, Belisle JT. Secretion of an acid phosphatase (SapM) by Mycobacterium tuberculosis that is similar to eukaryotic acid phosphatases. J Bacteriol. 2000;182:6850-3. https://doi.org/10.1128/JB.182.23.6850-6853.2000
Muthukrishnan G, Masters EA, Daiss JL, Schwarz EM. Mechanisms of immune evasion and bone tissue colonization that make Staphylococcus aureus the primary pathogen in osteomyelitis. Curr Osteoporos Rep. 2019;17:395-404. https://doi.org/10.1007/s11914-019-00548-4
Neal AL, Blackwell M, Akkari E, Guyomar C, Clark I, Hirsch PR. Phylogenetic distribution, biogeography and the effects of land management upon bacterial non-specific Acid phosphatase Gene diversity and abundance. Plant Soil. 2018;427:175-89. https://doi.org/10.1007/s11104-017-3301-2
Kelliher JL, Radin JN, Grim KP, Párraga-Solórzano PK, Degnan PH, Kehl-Fie TE. Acquisition of the phosphate transporter NptA enhances Staphylococcus aureus pathogenesis by improving phosphate uptake in divergent environments. Infect Immun. 2018;86. https://doi.org/10.1128/iai.00631-17
Kelliher JL, Leder-Macek AJ, Grudzinski KM, Radin JN, Kehl-Fie TE. Staphylococcus aureus preferentially liberates inorganic phosphate from organophosphates in environments where this nutrient is limiting. J Bacteriol. 2020;202. https://doi.org/10.1128/JB.00264-20
Some similar items:
- Gloria Heresi, Germán A. Contreras, Norma Pérez, James R. Murphy, Thomas G. Cleary, Empyema necessitans and acute osteomyelitis associated with community acquired methicillin resistant Staphylococcus aureus in an infant , Biomedica: Vol. 29 No. 4 (2009)
- César A. Arias, Marylin Hidalgo, Jinnethe Reyes, Ana María Cárdenas, Lorena Díaz, Sandra Ríncon, Natasha Vanegas, Paula Lucía Díaz, Elizabeth Castañeda, Resistance profiles to fluoroquinolones in clinical isolates of Gram positive cocci , Biomedica: Vol. 28 No. 2 (2008)
- Ana María Perilla, Camilo González, Sandra Liliana Valderrama, Natasha Vanegas, Bibiana Chavarro, Luis Carlos Triana, José Roberto Támara, Carlos Arturo Álvarez, Necrotizing pneumonia by community-acquired, methicillin-resistant Staphylococcus aureus in Colombia , Biomedica: Vol. 29 No. 4 (2009)
- Lino E. Torres, Lidice González, Karelia Melián, Jordis Alonso, Arlenis Moreno, Mayrín Hernández, Orlando Reyes, Ludisleydis Bermúdez, Javier Campos, Guillermo Pérez-Pérez, Boris L. Rodríguez, EPIYA motif patterns among Cuban Helicobacter pylori CagA positive strains , Biomedica: Vol. 32 No. 1 (2012)
- Aníbal A. Brizzio, Fabián A. Tedeschi, Fabián E. Zalazar, Multiplex PCR strategy for the simultaneous identification of Staphylococcus aureus and detection of staphylococcal enterotoxins in isolates from food poisoning outbreaks , Biomedica: Vol. 33 No. 1 (2013)
- Ana María García, María Virginia Villa, María Elena Escudero, Patricia Gómez, Margarita M. Vélez, María Isabel Múnera, Gloria Franco, Use of nasal mupirocin for Staphylococcus aureus: effect on nasal carriers and nosocomial infections. , Biomedica: Vol. 23 No. 2 (2003)
- Ivohne Fernanda Corrales, Jorge Alberto Cortés, María Lucía Mesa, Graciela Zamora, Sternal osteomyelitis and scrofuloderma due to BCG vaccination. , Biomedica: Vol. 23 No. 2 (2003)
- Sandra Huérfano, Maria Caridad Cepero, Elizabeth Castañeda, Phenotype characterization of environmental Cryptococcus neoformans isolates. , Biomedica: Vol. 23 No. 3 (2003)
- Leonardo F. Jurado, Martha I. Murcia, Patricia Hidalgo, John E. Leguizamón, Lorena R. González, Phenotypic and genotypic diagnosis of bone and miliary tuberculosis in an HIV+ patient in Bogotá, Colombia , Biomedica: Vol. 35 No. 1 (2015)
- Sara Catalina Penagos, Sebastián Gómez, Pablo Villa, Santiago Estrada, Carlos Andrés Agudelo, Osteomyelitis due to Yokenella regensburgei following craniotomy in an immunocompetent patient , Biomedica: Vol. 35 No. 4 (2015)
Copyright (c) 2023 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























