Expresión de alfa sinucleína en sangre y su relación con el estreñimiento crónico en población residente en Bogotá, D.C., con problemas de consumo de alcohol
Resumen
Introducción. El consumo excesivo de alcohol resulta en neuroadaptación, neurodegeneración y expresión diferencial de numerosos genes.
Objetivo. Determinar la relación entre la expresión del gen de la alfa sinucleína (SNCA) en sangre, las variantes de nucleótido único (Single Nucleotide Variant, SNV) en su región promotora y el estreñimiento crónico en personas con problemas de consumo de alcohol.
Materiales y métodos. La muestra estuvo conformada por 35 controles y 27 casos, seleccionados según el puntaje obtenido con la herramienta AUDIT. En el diagnóstico del estreñimiento se aplicaron los criterios de Roma IV. La extracción de ácidos nucleicos se hizo a partir de sangre periférica y se evaluó la expresión del gen mediante qPCR, la cuantificación proteica por ELISA y la presencia de SNV en la región promotora del gen por la secuenciación de Sanger.
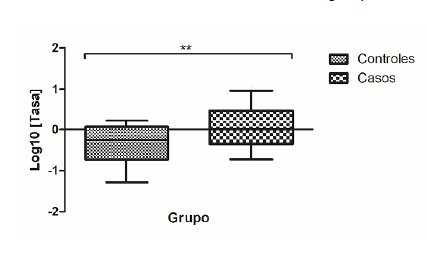
Resultados. Se observó sobreexpresión génica relativa de ARNm del gen SNCA en el grupo de casos sin relación con el estreñimiento crónico. Se evidenció un riesgo 4,8 veces mayor de presentar estreñimiento en el grupo de casos. Se encontraron nueve variantes de nucleótido simple en un segmento de la región promotora del gen rica en secuencias reguladoras CpG, con frecuencia similar entre los grupos, y se detectó una variante en la posición -2171 que no se encuentra reportada en GenBank para variantes clínicas y cuyo genotipo A/T se relacionó con el incremento de la expresión del ARNm del SNCA.
Conclusión. En personas con problemas de consumo de alcohol se evidenció la sobreexpresión del ARNm de alfa sinucleína, lo cual no se relacionó con el diagnóstico de estreñimiento crónico.
Descargas
Referencias bibliográficas
Organización Mundial de la Salud. Estrategia mundial para reducir el uso nocivo del alcohol. Ginebra: Organización Mundial de la Salud; 2010. p. 1-46. Fecha de consulta: 5 de enero de 2018. Disponible en: https://www.who.int/substance_abuse/activities/msbalcstrategyes.pdf
Observatorio de Drogas de Colombia. Estudio de consumo de sustancias psicoactivas en Colombia, 2013. p. 1-182. Fecha de consulta: 5 de enero de 2018. Disponible en: https://www.unodc.org/documents/colombia/2014/Julio/Estudio_de_Consumo_UNODC.pdf
Serecigni J. Neurobiología del alcoholismo. Psicología desde el Caribe. 2013;30:21-35.
Janeczek P, Lewohl J. The role of a-synuclein in the pathophysiology of alcoholism. Neurochem Int. 2013;63:154-62. https://doi.org/10.1016/j.neuint.2013.06.007
Swant J, Goodwin J, North A, Ali A, Gamble J, Chirwa S, et al. α-synuclein stimulates a dopamine transporter-dependent chloride current and modulates the activity of the transporter. J Biol Chem. 2011;286:43933-43. https://doi.org/10.1074/jbc.M111.241232
Liang T, Carr LG. Regulation of alpha-synuclein expression in alcohol-preferring and -non preferring rats. J Neurochem. 2006;99:470-82. https://doi.org/10.1111/j.1471-4159.2006.04111.x
Butler B, Saha K, Rana T, Becker JP, Sambo D, Davari P, et al. Dopamine transporter activity is modulated by a-synuclein. J Biol Chem. 2015;290:29542-54. https://doi.org/10.1074/jbc.M115.691592
Sui Y, Bullock K, Erickson M, Zhang J, Banks W. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides. 2014;62:197-202. https://doi.org/10.1016/j.peptides.2014.09.018
Lööv C, Scherzer CR, Hyman BT, Breakefield XO, Ingelsson M. α-synuclein in extracellular vesicles: Functional implications and diagnostic opportunities. Cell Mol Neurobiol. 2016;36:437-48. https://doi.org/10.1007/s10571-015-0317-0
Simonsen A, Kuiperij B, El-Agnaf A, Omar M, Engelborghs S, Herukka S-K, et al. The utility of α-synuclein as biofluid marker in neurodegenerative diseases: A systematic review of the literature. Biomark Med. 2016;10:19-34. https://doi.org/10.2217/BMM.14.105
Omar M, Agnaf E, Salem S, Paleologou K, Cooper L, Fullwood N, et al. Synuclein implicated in Parkinson’s disease is present in extracellular biological fluids including human plasma. FASEB J. 2003;17:1945-7. https://doi.org/10.1096/fj.03-0098fje
Henchcliffe C. Blood and cerebrospinal fluid markers in Parkinson’s disease: Current biomarker findings. Curr Biomark Find. 2015;5:1-11. https://doi.org/10.2147/CBF.S50424
Nakai M, Fujita M, Waragai M, Sugama S, Wei J, Akatsu H, et al. Expression of a-synuclein, a presynaptic protein implicated in Parkinson’s disease, in erythropoietic lineage. Biochem Biophys Res Commun. 2007;358:104-10. https://doi.org/10.1016/j.bbrc.2007.04.108
Abd-Elhadi S, Honig A, Simhi-Haham D, Schechter M, Linetsky E, Ben-Hur T, et al. Total and proteinase K-resistant α-synuclein levels in erythrocytes, determined by their ability to bind phospholipids, associate with Parkinson’s disease. Sci Rep. 2015;5:1-12. https://doi.org/10.1038/srep11120
Kang W, Chen W, Yang Q, Zhang L, Zhang L, Wang X, et al. Salivary total α-synuclein, oligomeric α-synuclein and SNCA variants in Parkinson’s disease patients. Sci Rep. 2016;6:1-8. https://doi.org/10.1038/srep28143
Wang X, Yu S, Li F, Feng T. Detection of α-synuclein oligomers in red blood cells as a potential biomarker of Parkinson’s disease. Neurosci Lett. 2015;599:115-9. https://doi.org/10.1016/j.neulet.2015.05.030
B̈nsch D, Reulbach U, Bayerlein K, Hillemacher T, Kornhuber J, Bleich S. Elevated alpha synuclein mRNA levels are associated with craving in patients with alcoholism. Biol sychiatry. 2004;56:984-6. https://doi.org/10.1016/j.biopsych.2004.09.016
Ziolkowska B, Gieryk A, Wawrzczak-Bargiela A, Krowka T, Kaminska D, Korkosz A, et al. α-Synuclein expression in the brain and blood during abstinence from chronic alcohol drinking in mice. Neuropharmacology. 2008;54:1239-46. https://doi.org/10.1016/j.neuropharm.2008.04.001
Walker S, Grant K. Peripheral blood α-synuclein mRNA levels are elevated in cynomolgus monkeys that chronically self-administer ethanol. Alcohol. 2006;38:1-4. https://doi.org/10.1016/j.alcohol.2006.03.008
Bönsch D, Lederer T, Reulbach U, Hothorn T, Kornhuber J, Bleich S. Joint analysis of the NACP-REP1 marker within the alpha synuclein gene concludes association with alcohol dependence. Hum Mol Genet. 2005;14:967-71. https://doi.org/10.1093/hmg/ddi090
Foroud T, Wetherill LF, Liang T, Dick DM, Hesselbrock V, Kramer J, et al. Association of alcohol craving with a-synuclein (SNCA). Alcohol Clin Exp Res. 2007;31:537-45. https://doi.org/10.1111/j.1530-0277.2007.00337.x
Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517-36. https://doi.org/10.1007/s00702-002-0808-2
Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27:716-9. https://doi.org/10.1002/mds.25020
Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson’s disease: The Thy1-aSyn (“Line 61”) mice. Neurotherapeutics. 2012;9:297-314. https://doi.org/10.1007/s13311-012-0104-2
Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, et al. Differential neuropathological alterations in transgenic mice expressing a-synuclein from the plateletderived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568-78. https://doi.org/10.1002/jnr.10231
Verbaan D, Marinus J, Visser M, Van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient reported autonomic symptoms in Parkinson disease. Neurology. 2007;69:333-41. https://doi.org/10.1212/01.wnl.0000266593.50534.e8
Wang L, Magen I, Yuan PQ, Subramaniam SR, Richter F, Chesselet MF, et al. Mice verexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil. 2012;24:1-12. https://doi.org/10.1111/j.1365-2982.2012.01974.x
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469-80.e12. https://doi.org/10.1016/j.cell.2016.11.018
Engen P, Green S, Voigt R, Forsyth C, Keshavarzian A. The gastrointestinal microbiome: Alcohol effects on the composition of intestinal microbiota. Alcohol Res. 2015;37:223-36. https://doi.org/10.13140/RG.2.1.4342.9285
Yan A, Fouts D, Brandl J, Starkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96-105. https://doi.org/10.1002/hep.24018
Domingo J. The new Rome criteria (IV) of functional digestive disorders in clinical practice. Med Clin (Barc). 2017;148:464-8. https://doi.org/10.1016/j.medcli.2016.12.020
Babor T, Higgins J, Saunders J, Monteiro M. Cuestionario de identificación de los transtornos debidos al consumo de alcohol. Ginebra: Organización Mundial de la Salud; 2001. p. 1-40.
Campo A, Villamil M, Herazo E. Confiabilidad y dimensionalidad del AUDIT en estudiantes de medicina. Psicología desde el Caribe. 2013;30:21-35.
Ospina J, Manrique F, Ariza N. Confiabilidad y dimensionalidad del cuestionario para identificación de trastornos debidos al consumo de alcohol (AUDIT) en estudiantes universitarios de Tunja (Colombia). Salud Uninorte. 2012;28:276-82.
Anderson P, Gual L, Colón J. Alcohol y atención primaria de la salud alcohol y atención primaria de la salud. Washington, D.C.: Organización Panamericana de la Salud; 2008.
Bönsch D, Greifenberg V, Bayerlein K, Biermann T, Reulbach U, Hillemacher T, et al. α-synuclein protein levels are increased in alcoholic patients and are linked to craving. Alcohol Clin Exp Res. 2005;29:763-5. https://doi.org/10.1097/01.ALC.0000164360.43907.24
Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805-8. https://doi.org/10.1126/science.1082320
Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635-46. https://doi.org/10.1016/j.molcel.2007.02.011
Doxakis E. Post-transcriptional regulation of a-synuclein expression by mir-7 and mir-153. J Biol Chem. 2010;285:12726-34. https://doi.org/10.1074/jbc.M109.086827
McMillan K, Murray T, Bengoa N, Cordero O, Cooper J, Buckley A, et al. Loss of microRNA-7 regulation leads to α-synuclein accumulation and dopaminergic neuronal loss in vivo. Mol Ther. 2017;25:2404-14. https://doi.org/10.1016/j.ymthe.2017.08.017
Wang L, Fleming S, Chesselet M, Taché Y. Abnormal colonic motility in mice overexpressing human wild-type α-synuclein. Neuroreport. 2008;19:873-6. https://doi.org/10.1097/WNR.0b013e3282ffda5e
Kuo Y, Li Z, Jiao Y, Gaborit N, Pani A, Morrison B, et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet. 2010;19:1633-50. https://doi.org/10.1097/WNR.0b013e3282ffda5e
Sharma A, Kurek J, Morgan JC, Wakade C, Rao SS. Constipation in Parkinson’s disease:
A nuisance or nuanced answer to the pathophysiological puzzle? Curr Gastroenterol Rep. 2018;20:1. https://doi.org/10.1007/s11894-018-0609-x
Abbott R, Petrovitch H, White L, Masaki K, Tanner C, Curb J, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456-62. https://doi.org/10.1212/WNL.58.5.838-a
Rodríguez O, Torres L, Meza K, López R, Ruiz H, Cosentino C. Estreñimiento como factor asociado a mayor severidad en pacientes con enfermedad de Parkinson del Instituto Nacional de Ciencias Neurológicas del Perú. Diagnóstico. 2019;57:180-3. https://doi.org/10.33734/diagnostico.v57i4.169
Leclercq S, De Timary P, Delzenne N, Stärkel P. The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl Psychiatry. 2017;7:e1048. https://doi.org/10.1038/tp.2017.15
Algunos artículos similares:
- Nelsy Loango, Martha Lucía Gallego, Beatriz Restrepo, Patricia Landázuri, Diferencias de sexo, edad y lípidos plasmáticos asociadas al polimorfismo de la apolipoproteína E en un grupo de escolares de Quindío, Colombia , Biomédica: Vol. 29 Núm. 3 (2009)
- Ney Callas, Elpidia Poveda, César Baracaldo, Patricia Hernández, Carlina Castillo, Martha Guerra, Polimorfismo genético de la apolipoproteína E en un grupo de escolares del centro-oriente colombiano: comparación con las concentraciones plasmáticas de lípidos y apolipoproteínas , Biomédica: Vol. 27 Núm. 4 (2007)
- Nelson Grisales, Omar Triana, Víctor Angulo, Nicolás Jaramillo, Gabriel Parra-Henao, Francisco Panzera, Andrés Gómez-Palacio, Diferenciación genética de tres poblaciones colombianas de Triatoma dimidiata (Latreille, 1811) mediante análisis molecular del gen mitocondrial ND4 , Biomédica: Vol. 30 Núm. 2 (2010)
- Claudia Ayala, Reggie García, Edith Cruz, Karol Prieto, Marta Bermúdez, Niveles de homocisteína y polimorfismos de los genes de la MTHFR y la CBS en pacientes colombianos con trombosis venosa superficial y profunda , Biomédica: Vol. 30 Núm. 2 (2010)
- Ismael Reyes, Raj Tiwari, Jan Geliebter, Niradiz Reyes, Análisis de micromatrices de ADN revela genes asociados a metástasis en líneas celulares de cáncer de próstata de rata , Biomédica: Vol. 27 Núm. 2 (2007)
- Olga María Moreno, Clara Isabel González, Diego Luis Saaibi, William Otero, Reynaldo Badillo, Javier Martín, Gerardo Ramírez, Polimorfismos de la región promotora del gen de la IL-10 y artritis reumatoide en una población colombiana , Biomédica: Vol. 27 Núm. 1 (2007)
- Vanihamín Domínguez, Itzen Aguiñiga, Leticia Moreno, Beatriz Torres, Edelmiro Santiago-Osorio, El caseinato de sodio incrementa número de linfocitos B en ratones , Biomédica: Vol. 37 Núm. 4 (2017)
- Luis A. Franco, Germán E. Matiz, Jairo Calle, Roberto Pinzón, Luis F. Ospina, Actividad antinflamatoria de extractos y fracciones obtenidas de cálices de Physalis peruviana L. , Biomédica: Vol. 27 Núm. 1 (2007)
- Carlos Isaza, Leonardo Beltrán, Julieta Henao, Gloria Porras, Alfredo Pinzón, Álvaro Vallejos, Jorge Machado, Factores genéticos y ambientales asociados con la respuesta a warfarina en pacientes colombianos , Biomédica: Vol. 30 Núm. 3 (2010)
- Ricardo A. Cifuentes, Emiliano Barreto, Selección supervisada de polimorfismos de nucleótido único en el síndrome de fatiga crónica , Biomédica: Vol. 31 Núm. 4 (2011)
| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |

























