Results of the national surveillance of antimicrobial resistance of Enterobacteriaceae and Gram negative bacilli in health care-associated infections in Colombia, 2012-2014
Abstract
Introduction: The Colombian National Antimicrobial Resistance Monitoring System for the surveillance of healthcare-associated infections was set up to meet this problem in the third quarter of 2012.
Objective: To describe resistance profiles and laboratory-based surveillance based on the information collected by the System.
Materials and methods: We conducted a retrospective and descriptive study of the information notified to the Colombian Public Health Surveillance System (Sivigila), and in the Whonet databases covering the period from July 2012 to December 2014 provided by the primary data-generating units in the country, as well as laboratory surveillance results from 1,642 phenotypic and genotypic tests on carbapenemase isolates (927 from Enterobacteriaceae, 614 from Pseudomonas spp. and 101 from Acinetobacter spp.).
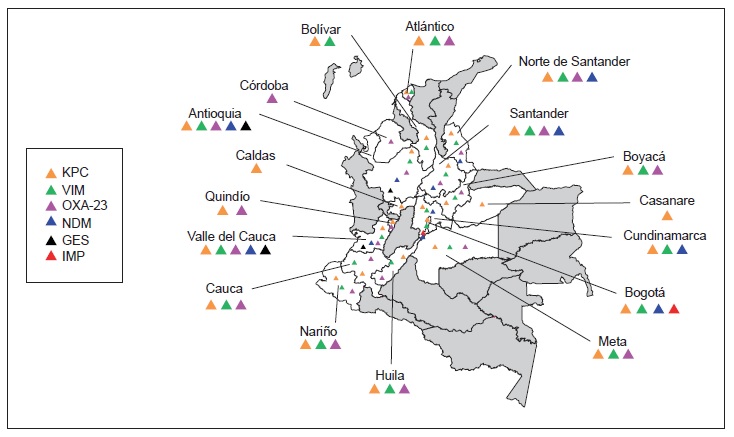
Results: There was a significant increase in Escherichia coli resistance to third-generation cephalosporins (reaching 26.3% in ICUs and 22.5% in other hospital wards), and Klebsiella pneumoniae resistance to ertapenem also increased (reaching 14.6% in ICUs). Acinetobacter baumannii carbapenem resistance exceeded 50% in ICUs whereas Pseudomonas aeruginosa had lower carbapenem resistance (38.8%). KPC (n = 574) and NDM (n=57) were the most frequently occurring carbapenemases in Enterobacteriaceae, VIM (n=229) and KPC (n=114) in P. aeruginosa, and OXA-23 in A. baumannii (n=87); several carbapenemase combinations were identified, KPC + VIM being the most common in Pseudomonas spp. and Enterobacteriaceae.
Conclusion: The data from the surveillance of healthcare-associated infections revealed significant carbapenem resistance profiles and antimicrobial resistance mechanisms circulating in Colombian healthcare institutions.
Downloads
References
Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and healthcare costs. Clin Infect Dis. 2006;15:S82-9. https://doi.org/10.1086/499406
Organización Mundial de la Salud. Estrategia mundial de la OMS para contener la resistencia a los antimicrobianos, 2001. Fecha de consulta: 19 de agosto de 2013. Disponible en: http://www.antibioticos.msssi.gob.es/PDF/resist_OMS_estrategia_mundial_contra_resistencias.pdf.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; approved standard. Twenty third information supplement. Document M100-S24. Wayne: CLSI; 2014.
Monteiro J, Widen RH, Pignatari AC, Kubasek C, Silbert S. Rapid detection of carbapenemase gene by multiplex real-time PCR. J Antimicrob Chemother. 2012; 67:906-9. https://doi.org/10.1093/jac/dkr563
Bonnin RA, Naas T, Poirel L, Nordmann P. Phenotypic, biochemical, and molecular techniques for detection of metallo-β-lactamase NDM in Acinetobacter baumannii. J Clin Microbiol. 2012;50:1419-21. https://doi.org/doi:10.1128/JCM.06276-11
Garza-Ramos U, Morfin-Otero R, Sader HS, Jones RN, Hernández E, Rodríguez-Noriega E, et al. Metallo-beta-lactamase gene bla(IMP-15) in a class 1 integron, In95, from Pseudomonas aeruginosa clinical isolates from a hospital in México. Antimicrob Agents Chemother. 2008;52:2943-6. https://doi.org/10.1128/AAC.00679-07
Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70:119-23. https://doi.org/10.1016/j.diagmicrobio.2010.12.002
Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27:351-3. https://doi.org/10.1016/j.ijantimicag.2006.01.004
Higgins PG, Lehmann M, Seifert H. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2010;35:305. https://doi.org/10.1016/j.ijantimicag.2009.10.014
Jones RN, Masterton R. Determining the value of anti-microbial surveillance programs. Diagn Microbiol Infect Dis. 2001;41:172-5. https://doi.org/10.1016/S0732-8893(01) 00318-2
Organización Panamericana de la Salud. Informe Anual de la Red de Monitoreo/Vigilancia de la Resistencia a los Antibió-ticos. 2010. Fecha de consulta: 2 de diciembre de 2016. Dis-ponible en: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=24101&Itemid=).
Villalobos A, Díaz M, Barrero L, Rivera S, Henríquez D, Villegas M, et al. Tendencias de los fenotipos de resistencia bacteriana en hospitales públicos y privados de alta complejidad de Colombia. Rev Panam Salud Pública. 2011;30:627-33. https://doi.org/10.1590/S1020-49892011001200022
Grupo para el Estudio de la Resistencia de los Antimicrobianos en Medellín. Microorganismos. Fecha de consulta: 20 de diciembre de 2015. Disponible en: http://www.grupogermen.org/microorganismos.html.
Secretaría Distrital de Salud Pública de Bogotá. Boletín IAAS 2014. Fecha de consulta: 11 de diciembre de 2016. Disponible en: http://www.saludcapital.gov.co/DSP/Resistencia%20Bacteriana/Boletines/Bolet%C3%ADn%20IAAS%202014.pdf.
Grupo para el Control de Resistencia Bacteriana de Bogotá (GREBO). Boletín informativo, años 2012-2014. Fecha de consulta: 11 de diciembre de 2016. Disponible en: http://www.grebo.org/grebo_site/jgrebo/index.php?option =com_content&view=article&id=73&Itemid=469
Villegas M, Lolans K, Correa A, Suárez C, López J, Vallejo M, et al. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother. 2006;50:2880-2. https://doi.org/10.1128/AAC. 00186-06
López JA, Correa A, Navon-Venezia S, Correa AL, Torres JA, Briceño DF, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect. 2011;17:52-6. https://doi.org/10. 1111/j.1469-0691.2010.03209.x
Mojica MF, Correa A, Vargas DA, Maya JJ, Montealegre MC, Rojas LJ, et al. Molecular correlates of the spread of KPC-producing Enterobacteriaceae in Colombia. Int J Antimicrob Agents. 2012;40:277-85. https://doi.org/10. 1016/j.ijantimicag.2012.05.006
Instituto de Salud Pública de Chile. Programa de Control de Infecciones Asociadas a la Atención en Salud. Boletín de Resistencia Antimicrobiana, 2015. Fecha de consulta: 9 de diciembre de 2016. Disponible en: http://www.ispch.cl/sites/default/files/BoletinRam-30112015A_0.pdf
Lefebvre B, Lévesque S, Bourgault AM, Mulvey MR, Mataseje, Boyd D, et al. Carbapenem non-susceptible Enterobacteriaceae in Quebec, Canada: Results of Labora-tory Surveillance Program (2010-2012). PLoS One. 2015;10: e0125076. https://doi.org/10.1371/journal.pone.0125076
Saavedra SY, Duarte C, González MN, Ovalle MV. Emer-gencia de Providencia rettgeri NDM-1 en dos departamentos de Colombia, 2012-2013. Enferm Infecc Microbiol Clin. 2015. https://doi.org/10.1016/j.eimc.2015.05.011
O’Mahony R, Quinn T, Drudy D, Walsh C, Whyte P, Mattar S. Antimicrobial resistance in nontyphoidal Salmonella from food sources in Colombia: Evidence for an unusual plasmid-localized class 1 integron in serotypes Typhimurium and Anatum. Microb Drug Resist. 2006;12:269-77. https://doi.org/10.1089/mdr.2006.12.269
Ribeiro VB, Falci DR, Rozales FP, Barth AL, Zavascki AP. Carbapenem-resistant GES-5-producing Klebsiella pneumoniae in Southern Brazil. Braz J Infect Dis. 2014; 18:231-2. https://doi.org/10.1016/j.bjid.2013.12.002
Boyd D, Taylor G, Fuller J, Bryce E, Embree J, Gravel D, et al. Complete sequence of four multidrug-resistant MOBQ1 Plasmids harboring blaGES-5 isolated from Escherichia coli and Serratia marcescens persisting in a Hospital in Canada. Canadian Nosocomial Infection Surveillance Program. Microb Drug Resist. 2015;21:253-60. https://doi.org/10. 1089/mdr.2014.0205
Silva F, Cifuentes M, Pinto E. Resultados de la vigilancia de susceptibilidad antimicrobiana en Chile: consolidando una red. Rev Chil Infect. 2011;28:19-27. https://doi.org/10.4067/S0716-10182011000100004
Correa A, Del Campo R, Perenguez M, Blanco VM, Rodríguez-Baños M, Pérez F, et al. Dissemination of high-risk clones of extensively drug-resistant Pseudomonas aeruginosa in Colombia. Antimicrob Agents Chemother. 2015;59:2421-5. https://doi.org/10.1128/AAC.03926-14
Vanegas JM, Cienfuegos AV, Ocampo AM, López L, del Corral H, Roncancio G, et al. Similar frequencies of Pseudomonas aeruginosa isolates producing KPC and VIM carbapenemases in diverse genetic clones at tertiary-care hospitals in Medellín, Colombia. J Clin Microbiol. 2014;52: 3978-86. https://doi.org/10.1128/JCM.01879-14
Dortet L, Poirel L, Nordmann P. Worldwide dissemina-tion of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int. 2014;2014:249.856. https://doi.org/10.1155/2014/249856
Villegas MV, Kattan JN, Correa A, Lolans K, Guzmán AM, Woodford N, et al. Dissemination of Acinetobacter baumannii clones with OXA-23 carbapenemase in Colombian hospitals. Antimicrob Agents Chemother. 2007;51:2001-4. https://doi.org/10.1128/AAC.00226-07
Saavedra SY, Núñez JC, Pulido IY, González EB, Valenzuela EM, Reguero MT, et al. Characterization of carbapenem-resistant Acinetobacter calcoaceticus--A. baumannii complex isolates in a third level hospital in Bogotá, Colombia. Int J Antimicrob Agents. 2008;31:389-91. https://doi.org/10.1016/j.ijantimicag.2007.12.008
Mostachio AK, Levin AS, Rizek C, Rossi F, Zerbini J, Costa SF. High prevalence of OXA-143 and alteration of outer membrane proteins in carbapenem-resistant Acinetobacter spp. isolates in Brazil. Int J Antimicrob Agents. 2012;39:396-401. https://doi.org/10.1016/j.ijantimicag.2012.01.021
Zander E, Bonnin RA, Seifert H, Higgins PG. Characteri-zation of blaOXA-143 variants in Acinetobacter baumannii and Acinetobacter pittii. Antimicrob Agents Chemother. 2014;58:2704-8. https://doi.org/10.1128/AAC.02618-13
Correa A, Montealegre MC, Mojica MF, Maya JJ, Rojas LJ, De La Cadena EP, et al. First report of a Pseudomonas aeruginosa isolate coharboring KPC and VIM carbapenemases. Antimicrob Agents Chemother. 2012;56: 5422-3. https://doi.org/10.1128/AAC.00695-12
Saavedra SY, Duarte C, González MN, Realpe ME. Carac-terización de aislamientos de Pseudomonas aeruginosa productores de carbapenemasas de siete departamentos de Colombia. Biomédica. 2014;34:217-23. https://doi.org/10. 7705/biomedica.v34i0.1685
Rojas LJ, Mojica MF, Blanco VM, Correa A, Montealegre MC, De La Cadena E, et al. Emergence of Klebsiella pneumoniae coharboring KPC and VIM carbapenemases in Colombia. Antimicrob Agents Chemother. 2013;57:1101-2. https://doi.org/10.1128/AAC.01666-12
Quiles MG, Rocchetti TT, Fehlberg LC, Kusano EJ, Chebabo A, Pereira RM, et al. Unusual association of NDM-1 with KPC-2 and armA among Brazilian Entero-bacteriaceae isolates. Braz J Med Biol Res. 2015;48:174-7. https://doi.org/10.1590/1414-431X20144154
Karthikeyan K, Thirunarayan MA, Krishnan P. Coexistence and armA in clinical isolates of Acinetobacter baumannii from India. J Antimicrobial Chemother. 2010;65:2253-4. https://doi.org/10.1093/jac/dkq273
Some similar items:
- Leidy González, Jorge Alberto Cortés, Systematic review of antimicrobial resistance in Enterobacteriaceae isolates from Colombian hospitals , Biomedica: Vol. 34 No. 2 (2014)
- Ana María Ocampo, Carlos Andrés Vargas, Patricia María Sierra, Astrid Vanessa Cienfuegos, Judy Natalia Jiménez, Molecular characterization of an outbreak of carbapenem-resistant Klebsiella pneumoniae in a tertiary care hospital in Medellín, Colombia , Biomedica: Vol. 35 No. 4 (2015)
- Johanna Marcela Vanegas, Luis Felipe Higuita, Carlos Andrés Vargas, Astrid Vanessa Cienfuegos, Erika Andrea Rodríguez, Gustavo Eduardo Roncancio, Judy Natalia Jiménez, Carbapenem-resistant Acinetobacter baumannii causing osteomyelitis and infections of skin and soft tissues in hospitals of Medellín, Colombia , Biomedica: Vol. 35 No. 4 (2015)
- Virgilio Galvis, Alejandro Tello, Alfredo Guerra, María Fernanda Acuña, Donaldo Villarreal, Antibiotic susceptibility patterns of bacteria isolated from keratitis and intraocular infections at Fundación Oftalmológica de Santander (FOSCAL), Floridablanca, Colombia , Biomedica: Vol. 34 (2014): Abril, Suplemento 1, Resistencia bacteriana
- César A. Arias, Marylin Hidalgo, Jinnethe Reyes, Ana María Cárdenas, Lorena Díaz, Sandra Ríncon, Natasha Vanegas, Paula Lucía Díaz, Elizabeth Castañeda, Resistance profiles to fluoroquinolones in clinical isolates of Gram positive cocci , Biomedica: Vol. 28 No. 2 (2008)
- Sandra Yamile Saavedra, Carolina Duarte, María Nilse González, María Elena Realpe, Characterization of isolates of carbapenemase-producing Pseudomonas aeruginosa from seven Colombian provinces , Biomedica: Vol. 34 (2014): Abril, Suplemento 1, Resistencia bacteriana
- Manuel Medell, Marcia Hart, María Luisa Batista, In vitro antimicrobial susceptibility in Enterococcus faecalis and Enterococcus faecium isolated from hospitalized patients , Biomedica: Vol. 34 (2014): Abril, Suplemento 1, Resistencia bacteriana
- Alfredo Prado, Nhora L. Arias, Mónica Chávez, Cristina E. Cabrera, Rómel F. Gómez, Phenotypic characterization of Acinetobacter baumannii isolates in a high-complexity healthcare institution in the city of Cali , Biomedica: Vol. 34 (2014): Abril, Suplemento 1, Resistencia bacteriana
- Marylin Hidalgo, Claudia Santos, Carolina Duarte, Elizabeth Castañeda, Clara Inés Agudelo, Increase in erythromycin-resistant Streptococcus pneumoniae in Colombia, 1994-2008 , Biomedica: Vol. 31 No. 1 (2011)
- Carmelo José Espinosa, Jorge Alberto Cortés, Juan Sebastián Castillo, Aura Lucía Leal, Systematic review of antimicrobial resistance among Gram positive cocci in hospitals in Colombia , Biomedica: Vol. 31 No. 1 (2011)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























