Sodium caseinate increases the number of B lymphocytes in mouse
Abstract
Introduction: Sodium caseinate, a casein salt, is a proinflammatory agent in mice, and it is able to induce granulopoiesis in vivo and to increase the production of cytokines, which is key for this biological process.
Objective: To assess whether sodium caseinate is able to induce a biological effect on cells from lymphoid origin and the production of cytokines involved in this lineage in vivo.
Materials and methods: We used female BALB /c mice from 8 to 12 weeks old. The animals were injected intraperitoneally (IP) with 1 ml of sodium caseinate (10% PBS w/v) four times every 48 hours. The B cell populations and the incorporation of BrdU were analyzed by flow cytometry. Detection of interleukin-7 was assessed by ELISA (Enzyme-Linked ImmunoSorbent Assay).
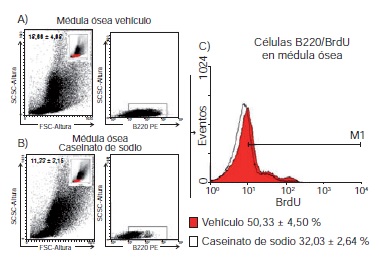
Results: We established that after intraperitoneal injection, the number of B lymphocytes 220+ from the spleen of mice treated with sodium caseinate increased compared to those that only received the vehicle (89.01±1.03 vs 75.66 ± 2.08), and the same was observed with the incorporation of BrdU in B220 + cells (38.59±4.48 vs 11.82±1.04 respectively). We also established that the concentration of interleukin-7 (IL-7) in the serum of mice treated with sodium caseinate increased compared to those that only received the vehicle (62.1 ± 17.5 vs 26.9 ± 4.4 pg/ml).
Conclusion: Sodium caseinate was able to increase the number of B lymphocytes in the spleen; it also induced IL-7 production, a cytokine that is key for the B cell lymphopoiesis.
Downloads
References
Lotem J, Sachs L. Control of in vivo differentiation of myeloid leukemic cells. Leukemia. 1988;2:24s-37s.
Lotem J, Sachs L. Independent regulation of myeloid cell growth and differentiation inducing proteins: In vivo regulation by compounds that induce inflammation. Int J Cancer. 1985;35:93-100.
Santiago-Osorio E, Mora L, Bautista M, Montesinos JJ, Martínez I, Ramos-Mandujano G, et al. Sodium caseinate induces secretion of macrophage colony-stimulating factor from neutrophils. Immunobiology. 2010;215:332-9. https://doi.org/10.1016/j.imbio.2009.03.003
Córdova-Galaviz Y, Ledesma-Martínez E, Aguíñiga-Sánchez I, Soldevila-Melgarejo G, Soto-Cruz I, Weiss-Steider B, et al. Sodium caseinate induces increased survival in leukaemic mouse J774 model. In Vivo. 2014;28: 819-25.
Santiago-Osorio E, Ledesma-Martínez E, Aguiñiga-Sánchez I, Poblano-Pérez I, Weiss-Steider B, Montesinos-Montesinos JJ, et al. Sodium caseinate (CasNa) induces mobilization of hematopoietic stem cells in a BALB/c mouse model. Med Sci Monit Basic Res. 2015;21:206-12. https://doi.org/10.12659/MSMBR.895442
Domínguez-Meléndez V, Silvestre-Santana O, Moreno-Fierros L, Aguiñiga-Sánchez I, Martínez L, Marroquín-Segura R, et al. Sodium caseinate induces mouse granulopoiesis. Inflamm Res. 2012;61:367-73. https://doi.org/10.1007/s00011-011-0421-7
Metcalf D, Robb L, Dunn AR, Mifsud S, Di Rago L. Role of granulocyte-macrophage colony-stimulating factor and granulocyte colony stimulating factor in the development of an acute neutrophil inflammatory response in mice. Blood. 1996;88:3755-64.
Wong CW, Seow HF, Liu AH, Husband AJ, Smithers GW, Watson DL. Modulation of immune responses by bovine beta-casein. Immunol Cell Biol. 1996;74:323-9. https://doi.org/10.1038/icb.1996.58
Tobita K, Kawahara T, Otani H. Bovine beta-casein (1-28), a casein phosphopeptide, enhances proliferation and IL-6 expression of mouse CD19+ cells via Toll-like receptor 4. J Agric Food Chem. 2006;54:8013-7. https://doi.org/10.1021/jf0610864
Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657-79. https://doi.org/10.1146/annurev.immunol.24.021605.090727
Sitnicka E, Bryder D, Theilgaard-Mönch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463-72. https://doi.org/10.1016/S1074-7613(02)00419-3
Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955-60. https://doi.org/10.1084/jem.180.5.1955
Instituto de Biotecnología, Universidad Nacional Autónoma de México. Norma Oficial Mexicana NOM-062-ZOO-1999. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Fecha de consulta: 15 de enero de 2010. Disponible en: http://www.ibt.unam.mx/computo/pdfs/bioterio.NOM-062.pdf
Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, et al. Characterization of purified intra-embryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci USA. 2005;102:134-9. https://doi.org/10.1073/pnas.0402270102
Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127-59. https://doi.org/10.1146/annurev.immunol.23.021704.115628
Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17: 399-433. https://doi.org/10.1146/annurev.immunol.17.1.399
Dias S, Silva H Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971-9. https://doi.org/10.1084/jem.20042393
Milne CD, Paige CJ. IL-7: A key regulator of B lympho-poiesis. Semin Immunol. 2006;18:20-30. https://doi.org/10. 1016/j.smim.2005.10.003
Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflam-mation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47-58. https://doi.org/10.1084/jem.20031104
Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771-80. https://doi.org/10.1084/jem.20041419
von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519-26. https://doi.org/10. 1084/jem.181.4.1519
Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94:1908-13
Inra CN, Zhou BO, Acar M, Murphy MM, Richardson J, Zhao Z, et al. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature. 2015;26:466-71. https://doi.org/10.1038/nature15530
Some similar items:
- Alejandra Catalina Vélez, Diana María Castaño, Rubén Darío Gómez, Julio César Orrego, Marcela Moncada, José Luis Franco, Common variable immunodeficiency: Clinical and immunological characterization of patients and homogeneous subgroup definition by means of B lymphocyte subpopulation typing , Biomedica: Vol. 35 No. 1 (2015)
- Marcel Marín, Yudy Alexandra Aguilar, José Robinson Ramírez, Omar Triana, Carlos Enrique Muskus, Molecular and immunological analyses suggest the absence of hydrophilic surface proteins in Leishmania (Viannia) panamensis , Biomedica: Vol. 28 No. 3 (2008)
- Luis A. Franco, Germán E. Matiz, Jairo Calle, Roberto Pinzón, Luis F. Ospina, Antiinflammatory activity of extracts and fractions obtained from Physalis peruviana L. calyces , Biomedica: Vol. 27 No. 1 (2007)
- Gloria Inés Múnera, Jairo Andrés Méndez, Gloria Janneth Rey, Serological, molecular and virological analyses associated with yellow fever surveillance in Colombia , Biomedica: Vol. 30 No. 3 (2010)
- Lucía Carolina Leal-Esteban, Jessica Lineth Rojas, Andrea Lizeth Jaimes, Juan David Montoya, Nilton Edu Montoya, Lily Leiva, Claudia Milena Trujillo-Vargas, An immunoenzymatic test for IgG antibody levels against 10 serotypes of Streptococcus pneumoniae , Biomedica: Vol. 32 No. 1 (2012)
- Juan Carlos Villa-Camacho, Juan Camilo Vargas-Zambrano, John Mario González, Flow cytometry model for the detection of neutralizing antibodies against of IFN-β , Biomedica: Vol. 32 No. 4 (2012)
- Luis Ángel Villar, Rosa Margarita Gélvez, Jairo Antonio Rodríguez, Doris Salgado, Beatriz Parra, Lyda Osorio, Irene Bosch, Biomarkers for the prognosis of severe dengue , Biomedica: Vol. 33 (2013): Suplemento 1, Fiebres hemorrágicas
- Piedad Agudelo, David Botero, Luis Guillermo Palacio, Evaluation of the ELISA method for diagnosis of human cysticercosis in an endemic region. , Biomedica: Vol. 25 No. 4 (2005)
- Viviana Marcela Rodríguez, Adriana Cuéllar, Lyda Marcela Cuspoca, Carmen Lucía Contreras, Marcela Mercado, Alberto Gómez, Phenotypical determinants of stem cell subpopulations derived from human umbilical cord blood. , Biomedica: Vol. 26 No. 1 (2006)
- Fabián Jaimes, Gisela de la Rosa, Anticoagulation and sepsis: the opportunity for a new use of heparin?. , Biomedica: Vol. 26 No. 1 (2006)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























