Genetic diversity and population structure of Anopheles triannulatus s. l. in the department of Córdoba, Colombia, using DNA barcoding
Abstract
Introduction: Anopheles triannulatus is not incriminated as a vector of malaria transmission in Colombia despite recent reports of infection with Plasmodium spp. in populations related to the northwestern and southeastern lineages. Genetic diversity can delimit information about gene flow and population differentiation in localities with malaria.
Objective: To estimate the genetic diversity of An. triannulatus in five municipalities with high and low incidence of malaria in the department of Córdoba.
Materials and methods: The entomological collections were done between August and November, 2016, in Tierralta, Puerto Libertador, Montelíbano, Sahagún, and Planeta Rica. We used the COI barcoding fragment as molecular marker. The genetic analysis included the estimation of genetic parameters such as the diversity haplotype, the genetic structure, the gene flow, the Tajima’s D test, the haplotype network, and the phylogenetic relationship.
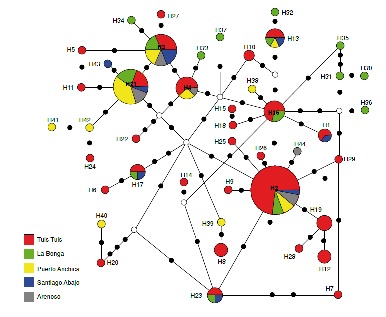
Results: We obtained 148 sequences with a length of 655 nucleotides of the COI gene, from which we derived 44 haplotypes. The H2 and H21 haplotypes were the most frequent in the populations. The values of the Tajima’s D test were negative and not significant (p>0.10). The genetic structure
index (FST=0.01427) and the gene flow (Nm=17.27) evidenced no differentiation between sampled populations due to the high exchange of migrants. Using phylogenetic inferences and the haplotype network, we identified one single species without geographic differentiation or lineages in the geographic range studied.
Conclusions: The genetic diversity calculated for An. triannulatus in this context indicated stable populations in constant exchange.
Downloads
References
Walter Reed Biosystematics Unit. Systematic catalog of Culicidae, 2017. Fecha de consulta: 16 de marzo de 2018. Disponible en: http://www.mosquitocatalog.org
Faran M, Linthicum K. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq Syst. 1981;13:1-81.
Chadde DD, Wilkerson R. Anopheles triannulatus (Neiva and Pinto): A new Anopheles record from Trinidad, West Indies. J Am Mosq Control Assoc. 2005;21:316-7. https://doi.org/10.2987/8756-971X(2005)21[316:ATNAPA]2.0.CO;2
González R, Carrejo N. Introducción al estudio taxonómico de Anopheles de Colombia: claves y notas de distribución. Segunda edicion. Cali: Universidad del Valle; 2009. p. 260.
Sinka M, Rubio-Palis Y, Manguin S, Patil A, Temperley W, Gething P, et al. The dominant Anopheles vectors of human malaria in the Americas: Occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:72. https://doi.org/10.1186/1756-3305-3-72
Galardo AK, Arruda M, Couto AR, Wirtz R, Lounibos LP, Zimmerman RH. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am J Trop Med Hyg. 2007;76:461-9. https://doi.org/10.4269/ajtmh.2007.76.461
Tadei WP, Thatcher BD. Malaria vectors in the Brazilian Amazon: Anopheles of the subgenus Nyssorhynchus. Rev Inst Med Trop São Paulo. 2000;42:87-94. https://doi.org/10.1590/S0036-46652000000200005
de Oliveira-Ferreira J, Lourenco-de-Oliveira R, Teva A, Deane LM, Daniel-Ribeiro CT. Natural malaria infections in Anophelines in Rondania State, Brazilian Amazon. Am J Trop Med Hyg. 1990;43:6-10.
Aramburú J, Ramal C, Witzig R. Malaria reemergence in the Peruvian Amazon Region. Emerg Infect Dis. 1999;5:209-15. https://doi.org/10.3201/eid0502.990204
Naranjo-Díaz N, Rosero DA, Rúa-Uribe G, Luckhart S, Correa MM. Abundance, behavior and entomological inoculation rates of anthropophilic anophelines from a primary Colombian malaria endemic area. Parasit Vectors. 2013;6:1-11. https://doi.org/10.1186/1756-3305-6-61
Rosero DN, Naranjo-Díaz N, Álvarez A, Cienfuegos C, Torres S, Luckhart S, et al. Colombian Anopheles triannulatus (Diptera: Culicidae) naturally infected with Plasmodium spp. ISRN Parasitol. 2013:2013:927453.https://doi.org/10.5402/2013/927453
Rosa-Freitas MG, Lourenço-de-Oliveira R, de Carvalho-Pinto CJ, Flores-Mendoza C, Fernandes T, Do-Nascimiento S. Anopheline species complexes in Brazil. Current knowledge of those related to malaria transmission. Mem Inst Oswaldo Cruz. 1998;93:651-5. https://doi.org/10.1590/S0074-02761998000500016
Silva-do-Nacimiento T, Lourenço-de-Oliveira R. Anopheles halophylus, a new species of the Subgenus Nyssorhynchus (Diptera: Culicidae) from Brazil. Mem Inst Oswaldo Cruz. 2002;97:801-11. https://doi.org/10.1590/S0074-02762002000600010
Rosa-Freitas MG, Tsouris P, Peterson AT, Honório NA, Barros FS, Aguiar DB, et al. An ecoregional classification for the state of Roraima, Brazil. The importance of landscape in malaria biology. Mem Inst Oswaldo Cruz. 2007;102:349-57. https://doi.org/10.1590/S0074-02762007005000052
Silva-do-Nascimento TF, Wilkerson RC, Monteiro FA. Molecular confirmation of the specific status of Anopheles halophylus (Diptera: Culicidae) and evidence of a new cryptic species within An. triannulatus in Central Brazil. J Med Entomol. 2006;43:455-9.
Silva-do-Nascimento TF, Damazio L, Pitaluga R, Peixoto AA, Lourenço-de-Oliveira R. Molecular divergence in the timeless and cpr genes among three sympatric cryptic species of the Anopheles triannulatus complex. Mem Inst Oswaldo Cruz. 2011;106:218-22. https://doi.org/10.1590/S0074-02762011000900027
Brochero H, Pareja PX, Ortiz G, Olano VA. Sitios de cría y actividad de picadura de especies de Anopheles en el municipio de Cimitarra, Santander, Colombia. Biomédica. 2006;26:269-77. https://doi.org/10.7705/biomedica.v26i2.1416
Gutiérrez LA, González JJ, Gómez GF, Castro MI, Rosero DA, Luckhart S, et al. Species composition and natural infectivity of anthropophilic Anopheles (Diptera: Culicidae) in Córdoba and Antioquia states in northwestern Colombia. Mem Inst Oswaldo Cruz. 2009;104:1117-24. https://doi.org/10.1590/S0074-02762009000800008
Gutiérrez LA, Naranjo NJ, Cienfuegos AV, Muskus CE, Luckhart S, Conn JE, et al. Population structure analyses and demographic history of the malaria vector Anopheles albimanus from the Caribbean and the Pacific regions of Colombia. Malar J. 2009;8:259. https://doi.org/10.1186/1475-2875-8-259
Rodríguez M, Pérez L, Caicedo JC, Prieto G, Arroyo JA, Kaur H, et al. Composition and biting activity of Anopheles (Diptera: Culicidae) in the Amazon Region of Colombia. J Med Entomol. 2009;46:307-15.
Rosero DA, Jaramillo LM, Gutiérrez LA, Conn JE, Correa MM. Genetic diversity of Anopheles triannulatus s.l. (Diptera: Culicidae) from Northwestern and Southeastern Colombia. Am J Trop Med Hyg. 2012;87:910-20. https://doi.org/10.4269/ajtmh.2012.12-0285
Collins FH, Méndez A, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V. Ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 1987;37:37-41. https://doi.org/10.4269/ajtmh.1987.37.37
Atencia M, Pérez M, Jaramillo M, Caldera S, Bejarano E. Primer reporte de la mutación F1534C asociada con resistencia cruzada a DDT y piretroides en Aedes aegypti en Colombia. Biomédica. 2016;36:432-7. https://doi.org/10.7705/biomedica.v36i3.2834
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294-9.
Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870-4. https://doi.org/10.1093/molbev/msw054
Thompson J, Higgins D, Gibson T. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673-80.
Altschup S, Gish W, Miller W, Myers E, Lipman D. Basic local alignment search tool department of computer science. J Mol Biol. 1990;215:403-10. https://doi.org/10.1016/S0022-2836(05)80360-2
Ratnasingham S, Hebert PDN. Bold: The Barcode of Life Data System (http://www.barcodinglife.org). Molecular Ecology Notes. 2007;7:355-64. https://doi.org/10.1111/j.1471-8286.2007.01678.x
Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451-2. https://doi.org/10.1093/bioinformatics/btp187
Excoffier L, Lischer HE. Arlequin Suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10:564-7. https://doi.org/10.1111/j.1755-0998.2010.02847.x
Fluxus Technology. Network 5.0. Clare: Fluxus Technology Ltd.; 2017.
Badelt H, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 1999;16:37-48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Posada D. jModelTest: Phylogenetic model averaging. Mol Biol Evol. 2008;25:1253-6. https://doi.org/10.1093/molbev/msn083
Ronquist F, Huelsenbeck J. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572-4. https://doi.org/10.1093/bioinformatics/btg180
Gutiérrez LA, Gómez GF, González JJ, Castro MI, Luckhart S, Conn JE, et al. Microgeographic genetic variation of the malaria vector Anopheles darlingi Root (Diptera: Culicidae) from Córdoba and Antioquia, Colombia. Am J Trop Med Hyg. 2010;83:38-47. https://doi.org/10.4269/ajtmh.2010.09-0381
Jaramillo LM, Gutiérrez LA, Luckhart S, Conn JE, Correa MM. Molecular evidence for a single taxon, Anopheles nuneztovari s.l., from two endemic malaria regions in Colombia. Mem Inst Oswaldo Cruz. 2011;106:1017-23. https://doi.org/10.1590/S0074-02762011000800020
Pedro PM, Uezu A, Sallum MA. Concordant phylogeographies of 2 malaria vectors attest to common spatial and demographic histories. J Hered. 2010;101:618-27. https://doi.org/10.1093/jhered/esq054
Urrea PA, Correa M, Naranjo-Díaz N. Variabilidad genética de Anopheles punctimacula s.l. en dos localidades de la zona endémica para la malaria: el Bajo Cauca y Alto Sinú. Hechos Microbiológicos. 2014;5:51-62.
Dai Y, Huang X, Cheng P, Liu L, Wang H, Wang H, et al. Development of insecticide resistance in malaria vector Anopheles sinensis populations from Shandong province in China. Malar J. 2015;14:62. https://doi.org/10.1186/s12936-015-0592-8
Scarpassa VM, Cunha-Machado AS, Saraiva JF. Evidence of new species for malaria vector Anopheles nuneztovari sensu lato in the Brazilian Amazon region. Malar J. 2016;15:205. https://doi.org/10.1186/s12936-016-1217-6
McKeon SN, Lehr M, Wilkerson RC, Ruiz JF, Sallum M, Lima JB, et al. Lineage divergence detected in the malaria vector Anopheles marajoara (Diptera: Culicidae) in Amazonian Brazil. Malar J. 2010;9:271. https://doi.org/10.1186/1475-2875-9-271
Ahumada ML, Orjuela LI, Pareja PX, Conde M, Cabarcas DM, Cubillos EFG, et al. Spatial distributions of Anopheles species in relation to malaria incidence at 70 localities in the highly endemic Northwest and South Pacific coast regions of Colombia. Malar J. 2016;15:407. https://doi.org/10.1590/S0074-02762009000800008
Some similar items:
- Yazmin Rocío Arias, Karime Osorio-Arango, Brayan Bayona, Guadalupe Ercilla, Mauricio Beltrán-Durán, Determination of HLA-A, -B and -DRB1 polymorphism in brain dead organ donors representative of the Colombian general population, 2007-2014 , Biomedica: Vol. 37 No. 2 (2017)
- Jorge A. Vega, Simón Villegas-Ospina, Wbeimar Aguilar-Jiménez, María T. Rugeles, Gabriel Bedoya, Wildeman Zapata, Haplotypes in CCR5-CCR2, CCL3 and CCL5 are associated with natural resistance to HIV-1 infection in a Colombian cohort , Biomedica: Vol. 37 No. 2 (2017)
- Juan J. Yunis, Luis E. Acevedo, David S. Campo, Emilio J. Yunis, Geno-geographic origin of Y-specific STR haplotypes in a sample of Caucasian-Mestizo and African-descent male individuals from Colombia , Biomedica: Vol. 33 No. 3 (2013)
- Sandra Milena Barrera, Manuel Alberto Pérez, Angélica Knudson, Rubén Santiago Nicholls, Ángela Patricia Guerra, Genotypic survery of Plasmodium falciparum based on the msp1, msp2 and glurp genes by multiplex PCR , Biomedica: Vol. 30 No. 4 (2010)
- Raúl Murillo, Ricardo Cendales, Carolina Wiesner, Marion Piñeros, Sandra Tovar, Effectiveness of cytology-based cervical cancer screening in the Colombian health system , Biomedica: Vol. 29 No. 3 (2009)
- Sandra Lorena Girón, Julio César Mateus, Fabián Méndez, Impact of an open waste disposal site on the occurrence of respiratory symptoms and on health care costs of children , Biomedica: Vol. 29 No. 3 (2009)
- Jaime E. Bernal, Martha Lucía Tamayo , Ignacio Briceño , Escilda Benavides , Newborn screening in Colombia: The experience of a private program in Bogotá , Biomedica: Vol. 44 No. 1 (2024)
- Andrés Páez, Gloria Rey, Carlos Agudelo, Alvaro Dulce, Edgar Parra, Hernando Díaz-Granados, Damaris Heredia, Luis Polo, Outbreak of urban rabies transmitted by dogs in Santa Marta, northern Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Patricia Escobar, Katherine Paola Luna, Indira Paola Hernández, César Mauricio Rueda, María Magdalena Zorro, Simon L. Croft, In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole , Biomedica: Vol. 29 No. 3 (2009)
- Mauricio Beltrán, María Cristina Navas, María Patricia Arbeláez, Jorge Donado, Sergio Jaramillo, Fernando De la Hoz, Cecilia Estrada, Lucía del Pilar Cortés, Amalia de Maldonado, Gloria Rey, Seroprevalence of hepatitis B virus and human immunodeficiency virus infection in a population of multiply-transfused patients in Colombia , Biomedica: Vol. 29 No. 2 (2009)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























