Adverse treatment outcomes in multidrug resistant tuberculosis go beyond the microbe-drug interaction: Results of a multiple correspondence analysis.
Abstract
Introduction: Multidrug-resistant tuberculosis treatment is effective in 50% of patients due to several factors including antibiotic susceptibility of the microorganism, adverse treatment reactions, social factors, and associated comorbidities.
Objectives: In this study, we describe the demographics, clinical characteristics, and factors associated with treatment outcomes in multidrug-resistant tuberculosis (MDR-TB) patients in Medellín, Colombia.
Materials and methods: We conducted a retrospective analysis using data from patients diagnosed with MDR-TB attending Hospital La María in Medellín, Colombia, for treatment between 2010 and 2015. Patients were categorized as having successful (cured) or poor (failure, lost to follow-up, and death) treatment outcomes. Associations between demographic, clinical factors, laboratory results, treatment outcomes, and follow-up information were evaluated by univariate, multivariate, and multiple correspondence analyses.
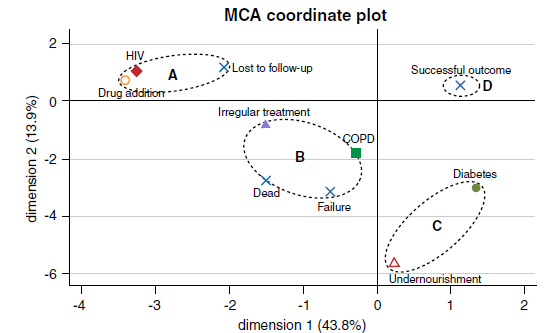
Results: Of the 128 patients with MDR-TB, 77 (60%) had successful outcomes. Of those with poor outcomes, 26 were lost to follow-up, 15 died, and 10 were treatment failures. Irregular treatment, the presence of comorbidities, and positive cultures after more than two months of treatment were associated with poor outcomes compared to successful ones (p<0.05 for all). The multiple correspondence analyses grouped patients who were lost to follow-up, had HIV, and drug addiction, as well as patients with treatment failure, irregular treatment, and chronic obstructive pulmonary disease.
Conclusion: The recognition of factors affecting treatment is essential and was associated with treatment outcomes in this series of patients. Early identification of these factors should increase the rates of treatment success and contribute to MDR-TB control.
Downloads
References
World Health Organization. Global tuberculosis report – 2012. Accessed on: December 5, 2018. Available at: https://apps.who.int/iris/handle/10665/75938
World Health Organization. Global tuberculosis report – 2017. Accessed on: November 8, 2018. Available at: https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf
Lucía M, Martínez O, Enrique M, Durán M, Pacheco-García OE, Bonilla HQ, et al. Protocolo de vigilancia en salud publica tuberculosis – 2016. Accessed on: November 12, 2018. Available at: http://www.saludpereira.gov.co/medios/Tuberculosis_PROTOCOLO_farmacoresistente2016.pdf
Secretaría de Salud y Protección Social de Antioquia. Situación de la tuberculosis en el departamento de Antioquia 2015-2016. Boletín Información para la Acción - 2017. Accessed on: October 5, 2018. Available at: https://www.dssa.gov.co/images/Bia_%20Tuberculosis_Mayo2017.pdf
Gupta R, Cegielski JP, Espinal MA, Henkens M, Kim JY, Lee JW, et al. Increasing transparency in partnerships for health--introducing the Green Light Committee. Trop Med Int Health. 2002;7:970-6. https://doi.org/10.1046/j.1365-3156.2002.00960.x
World Health Organization. Definitions and reporting framework for tuberculosis 2013 revision –2014. Accessed on: July 11, 2018. Available at: https://www.who.int/tb/publications/definitions/en/
Pfyffer GE, Palicova F. Mycobacterium: General characteristics, laboratory detection, and staining procedures. Manual of clinical microbiology, 10th edition. Washington, D.C.: American Society of Microbiology; 2011. p. 472-502.
Robledo J, Mejía GI, Paniagua L, Martín A, Guzmán A. Rapid detection of rifampicin and isoniazid resistance in Mycobacterium tuberculosis by the direct thin-layer agar method. Int J Tuberc Lung Dis. 2008;12:1482-4.
Rodrigues C, Jani J, Shenai S, Thakkar P, Siddiqi S, Mehta A. Drug susceptibility testing of Mycobacterium tuberculosis against second-line drugs using the Bactec MGIT 960 System. Int J Tuberc Lung Dis. 2008;12:1449-55.
Clinical and Laboratory Standards Institute. M24-A2 Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic actinomycetes. 2nd edition. Wayne (PA): Clinical and Laboratory Standards Institute; 2011.
Rusch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol. 2006;44:688–92. https://doi.org/10.1128/JCM.44.3.688-692.2006
Instituto Nacional de Salud. Lineamientos para el manejo programático de los pacientes con tuberculosis farmacorresistente – 2013. Accessed on: July 15, 2018. Available at: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/ET/lineamientos-tbfarmacorresistente.pdf
Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrugresistant tuberculosis: A systematic review and meta-analysis. PLoS One. 2009;4:e6914. https://doi.org/10.1371/journal.pone.0006914
Bolhuis MS, Akkerman OW, Sturkenboom MG, de Lange WC, van der Werf TS, Alffenaar J-WC. Individualized treatment of multidrug-resistant tuberculosis using therapeutic drug monitoring. Int J Mycobacteriol. 2016;5:S44-5. https://doi.org/10.1016/j.ijmyco.2016.07.003
Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: Systematic review. Trop Med Int Health. 2010;15:1-15. https://doi.org/10.1111/j.1365-3156.2010.02508.x
Kuchukhidze G, Kumar AM, de Colombani P, Khogali M, Nanava U, Blumberg HM, et al. Risk factors associated with loss to follow-up among multidrug-resistant tuberculosis patients in Georgia. Public Health Action. 2014;4:S41-6. https://doi.org/10.5588/pha.14.0048
Surya PI, Davies FL, Bruchfeld J, Hak E, Alffenaar J-W. Risk factors of multidrug-resistant tuberculosis: A global systematic review and meta-analysis. J Infect. 2018;77:469-78. https://doi.org/10.1016/j.jinf.2018.10.004
Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: A retrospective cohort study. Lancet. 2010;375:1798-807. https://doi.org/10.1016/S0140-6736(10)60492-8
Tang S, Tan S, Yao L, Li F, Li L, Guo X, et al. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: Retrospective multi-center investigation. PLoS One. 2013;8:e82943. https://doi.org/10.1371/journal.pone.0082943
Caminero JA. Treatment of multidrug-resistant tuberculosis: Evidence and controversies. Int J Tuberc Lung Dis. 2006;10:829-37.
Marrone MT, Venkataramanan V, Goodman M, Hill AC, Jereb JA, Mase SR. Surgical interventions for drug-resistant tuberculosis: A systematic review and meta-analysis. Int J Tuberc Lung Dis. 2013;17:6-16.
Jain K, Desai M, Solanki R, Dikshit RK. Treatment outcome of standardized regimen in patients with multidrug-resistant tuberculosis. J Pharmacol Pharmacother. 2014;5:145-9. https://doi.org/10.4103/0976-500X.130062
Kurbatova EV, Cegielski JP, Lienhardt C, Akksilp R, Bayona J, Becerra MC, et al. Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: A secondary analysis of data from two observational cohort studies. Lancet Respir Med. 2015;3:201-9. https://doi.org/10.1016/S2213-2600(15)00036-3
World Health Organization. The shorter MDR-TB regimen – 2016. Accessed on: December 3, 2018. Available at: https://www.who.int/tb/Short_MDR_regimen_factsheet.pdf
Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15:871-85. https://doi.org/10.5588/ijtld.10.0352
Whitfield MG, Soeters HM, Warren RM, York T, Sampson SL, Streicher EM, et al. A global perspective on pyrazinamide resistance: Systematic review and meta-analysis. PLoS One. 2015;10:e0133869. https://doi.org/10.1371/journal.pone.0133869
Ghafoor T, Ikram A, Abbasi SA, Zaman G, Ayyub M, Palomino JC, et al. Sensitivity pattern of second line anti-tuberculosis drugs against clinical isolates of multidrug-resistant Mycobacterium tuberculosis. J Coll Physicians Surg Pak. 2015;25:250-3.
Simpson G, Coulter C, Weston J, Knight T, Carter R, Vincent S, et al. Resistance patterns of multidrug-resistant tuberculosis in Western Province, Papua New Guinea. Int J Tuberc Lung Dis. 2011;15:551–2. https://doi.org/10.5588/ijtld.10.0347
Rueda J, Realpe T, Mejía GI, Zapata E, Rozo JC, Ferro BE, et al. Genotypic analysis of genes associated with independent resistance and cross-resistance to isoniazid and ethionamide in Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother. 2015;59:7805-10. https://doi.org/10.1128/AAC.01028-15
Pietersen E, Ignatius E, Streicher EM, Mastrapa B, Padanilam X, Pooran A, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: A cohort study. Lancet. 2014;383:1230-9. https://doi.org/10.1016/S0140-6736(13)62675-6
Some similar items:
- Ana María Vásquez, Felipe Sanín, Luis Gonzalo Álvarez, Alberto Tobón, Alexandra Ríos, Silvia Blair, Therapeutic efficacy of a regimen of artesunate-mefloquine-primaquine treatment for Plasmodium falciparum malaria and treatment effects on gametocytic development , Biomedica: Vol. 29 No. 2 (2009)
- Ricardo Sánchez, Gerardo Téllez, Luis Eduardo Jaramillo, Age of onset symptoms and gender in schizophrenic spectrum disorders , Biomedica: Vol. 32 No. 2 (2012)
- Mónica Alejandra Bernal-Vargas, Jorge Alberto Cortés, Ricardo Sánchez, Cross-cultural adaptation of the community-acquired pneumonia score questionnaire in patients with mild-to-moderate pneumonia in Colombia , Biomedica: Vol. 37 No. 1 (2017)
- Claudia Llerena, Angie Zabaleta, Angélica Valbuena, Martha Murcia, Prevalence of Mycobacterium tuberculosis resistance to quinolones and injectables in Colombia, 2012-2013 , Biomedica: Vol. 37 No. 1 (2017)
- Nelson José Alvis-Zakzuk, María de los Ángeles Carrasquilla, Verónica Jhajaira Gómez, Jaime Robledo, Nelson Rafael Alvis-Guzmán, José Mauricio Hernández, Diagnostic accuracy of three technologies for the diagnosis of multi-drug resistant tuberculosis , Biomedica: Vol. 37 No. 3 (2017)
- Angie Paola Zabaleta, Claudia Llerena, Angélica Valbuena, Resistant tuberculosis in children under 15 years of age, Colombia 2010-2015 , Biomedica: Vol. 39 No. 2 (2019)
- Angie Zabaleta, Claudia Llerena, Extensively resistant tuberculosis, Colombia, 2006-2016 , Biomedica: Vol. 39 No. 4 (2019)
- Lina Sofía Morón-Duarte, Kelly Rocío Chacón, Maria Paula, Ilich Herbert, Nancy, Efficacy and safety of four COVID-19 vaccines in preventing SARS-CoV-2 infection: A rapid review , Biomedica: Vol. 42 No. Sp. 2 (2022): Covid-19
- Edwin Pulido, Mauricio González , Óscar Gamboa , Jairo Bonilla , Joaquín Luna, Raúl Murillo, Effectiveness of cryotherapy delivered by nurses for treatment of cervical preneoplasic lesions , Biomedica: Vol. 43 No. Sp. 3 (2023): Enfermedades crónicas no transmisibles
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























