Local deliberative approach to the bioethical controversies: An opportunity for the proper implementation of neonatal screening
Abstract
Introduction: The controversial characteristics of neonatal screening influenced by bioethical considerations make its implementation complex. Colombia is not an exception in this sense and local circumstances complicate the panorama.
Objective: To establish how bioethical controversies on neonatal screening are approached at a local level as a basis for deliberating on the must-be of this activity in Colombia.
Materials and methods: A survey immersed in an interpretative investigation with descriptive and deliberative components of analysis was applied to approach the values exposed by officials of the Colombian Instituto Nacional de Salud.
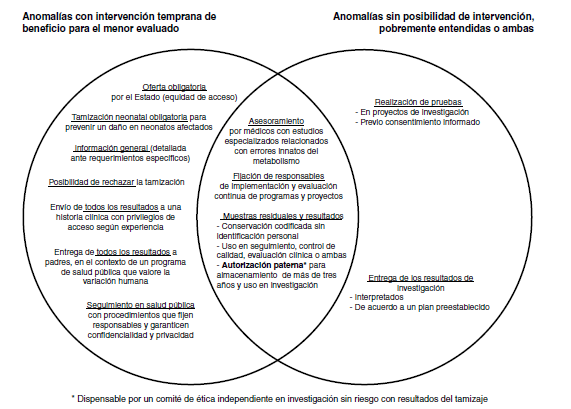
Results: The compulsory offer of screening by the nation, regardless of its opportunity cost and the consent for the use in research of results and residual samples, were not controversial, but, in contrast, the type of information and the consent to authorize screening did arise controversy. The more experienced officials preferred mandatory screening (17.7 vs. 11.79 years on average, p=0.007). Surprisingly, despite the risk of discrimination, keeping the neonate as the purpose, there was agreement on giving all the information to parents and medical records. Another controversial aspect was the follow-up of cases without hiding their identification where officials with more experience in bioethical aspects preferred the use of codes (4.5 vs. 1.26 years on average, p=0.009). In this context, strategies such as informed dissent, specialized advice or public health programs that appreciate diversity would allow to rescue even seemingly opposite values.
Conclusion: A local approach regarding what ought to be in neonatal screening based on a deliberative bioethical perspective allowed to present an implementation proposal for this activity
Downloads
References
Cifuentes RA. Consideraciones bioéticas del tamizaje neonatal: pautas para su regulación integral. Rev Latinoam Bioet. 2015;16:154-73. https://doi.org/10.18359/rlbi.1445
Fleischman AR, Lin BK, Howse JL. A commentary on the President’s Council on Bioethics report: The changing moral focus of newborn screening. Genet Med. 2009;11:507-9. https://doi.org/10.1097/GIM.0b013e3181a9d66c
Cifuentes RA. Bioética y políticas públicas de tamización neonatal en los Estados Unidos, el Reino Unido y Colombia. Biomédica. 2019;39:132-46. https://doi.org/10.7705/biomedica.v39i1.3906
Grosse SD, Olney RS, Baily MA. The cost effectiveness of universal versus selective newborn screening for sickle cell disease in the US and the UK: A critique. Appl Health Econ Health Policy. 2005;4:239-47. https://doi.org/10.2165/00148365-200504040-00006
President’s Council on Bioethics. The Changing Moral Focus of Newborn Screening 2008. Washington, D.C. Fecha de consulta: 7 de noviembre de 2014. Disponible en:
Chadwick R, ten Have H, Husted J, Levitt M, McGleenan T, Shickle D, et al. Genetic screening and ethics: European perspectives. J Med Philos. 1998;23:255-73. https://doi.org/10.1076/jmep.23.3.255.2580
Public Health England. Data Collection and Performance Analysis Report Newborn Blood Spot Screening in the UK 2014. Fecha de consulta: 17 de octubre de 2015. Disponible en: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/456183/2013-14_Data_Collection_and_Performance_Analysis_report.pdf
Lewis MH, Goldenberg A, Anderson R, Rothwell E, Botkin J. State laws regarding the retention and use of residual newborn screening blood samples. Pediatrics. 2011;127:703-12. https://doi.org/10.1542/peds.2010-1468
Therrell BL Jr., Hannon WH, Bailey DB Jr., Goldman EB, Monaco J, Norgaard-Pedersen B, et al. Committee report: Considerations and recommendations for national guidance regarding the retention and use of residual dried blood spot specimens after newborn screening. Genet Med. 2011;13:621-4. https://doi.org/10.1097/GIM.0b013e3182147639
Friedman L, Saal H, David K, Anderson R. Technical report: Ethical and policy issues in genetic testing and screening of children. Genet Med. 2013;15:234-45. https://doi.org/10.1038/gim.2012.176
Taylor-Phillips S, Boardman F, Seedat F, Hipwell A, Gale N, Clarke A, et al. The ethical, social and legal issues with expanding the newborn blood spot test 2014. Fecha de consulta: 17 de octubre de 2015. Disponible en: http://legacy.screening.nhs.uk/policydb_download.php?doc=455
Kemper AR, Fant KE, Clark SJ. Informing parents about newborn screening. Public Health Nurs. 2005;22:332-8. https://doi.org/10.1111/j.0737-1209.2005.220408.x
Parker H, Qureshi N, Ulph F, Kai J. Imparting carrier status results detected by universal newborn screening for sickle cell and cystic fibrosis in England: A qualitative study of current practice and policy challenges. BMC Health Serv Res. 2007;7:203. https://doi.org/10.1186/1472-6963-7-203
Elliman D. Ethical aspects of the expansion of neonatal screening programme due to technological advances. Clin Chem Lab Med. 2012;50:999-1002. https://doi.org/10.1515/cclm.2011.761
Congreso de la República de Colombia. Ley 1980 de 2019. Fecha de consulta: 29 de julio de 2019. Disponible en: http://www.camara.gov.co/tamizaje-neonatal-0
Ruiz J, Romero R, Buitrago A. Guía de práctica clínica. Detección de anomalías congénitas en el recién nacido 2013. Bogotá, D.C.: Ministerio de Salud y Protección Social-Colciencias. Fecha de consulta: 13 de marzo de 2017. Disponible en: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/INEC/IETS/GPC_Ptes_AC.pdf
Congreso de la República de Colombia. Ley 1098 de 2006. Fecha de consulta: 16 de junio de 2016. Disponible en: https://www.minsalud.gov.co/Normatividad_Nuevo/Ley%201980%20de%202019.pdf
Ministerio de Salud de Colombia. Resolución 13437 de 1991. Fecha de consulta: 31 de agosto de 2014. Disponible en: https://www.minsalud.gov.co/Normatividad_Nuevo/RESOLUCI%C3%93N%2013437%20DE%201991.pdf
Ministerio de la Protección Social de Colombia. Resolución 3518 de 2006. Fecha de consulta: 15 de marzo de 2017. Disponible en: http://www.ins.gov.co/normatividad/Decretos/DECRETO%203518%20DE%202006.pdf
Buendía L, González D, Gutiérrez J, Pelgajar M. Modelos de análisis de la investigación educativa. Sevilla: Ediciones Alfar; 1999. p. 34-9.
Gracia D. Ethical case deliberation and decision making. Med Health Care Philos. 2003;6:227-33. https://doi.org/10.1023/a:1025969701538
Gracia D. Procedimiento o método de toma de decisiones: Organización Médica Colegial de España. Fecha de consulta: 15 de marzo de 2017. Disponible en: https://www.google.com.co/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwjEzbGXmL_PAhWEHx4KHVAxDZUQFggaMAA&url=http%3A%2F%2Fwww.ffomc.
org%2FCursosCampus%2FExperto_Etica_Medica%2FU7_Procedimiento%2520o%2520metodo%2520de%2520toma%2520de%2520decisiones.pdf&usg=AFQjCNGI_o03qZi0WiTXBwjXmwiFaZtMtw&sig2=wJ_Zm6CVSB6hx-tg92n8Og
UK Newborn Screening Programme Centre. Health Professional Handbook A guide to newborn blood spot screening for healthcare professionals 2012. Fecha de consulta: 28 de octubre de 2015. Disponible en: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/390977/Health_Professional_Handbook_2012_v1.0_December_2012.pdf
Fost N. Informed consent should be a required element for newborn screening, even for disorders with high benefit-risk ratios. J Law Med Ethics. 2016;44:241-55. https://doi.org/10.1177/1073110516654118
Raffan E, Semple RK. Next generation sequencing--implications for clinical practice. Br Med Bull. 2011;99:53-71. https://doi.org/10.1093/bmb/ldr029
Kerruish NJ, Robertson SP. Newborn screening: New developments, new dilemmas. J Med Ethics. 2005;31:393-8. https://doi.org/10.1136/jme.2004.008219
Congreso de la República de Colombia. Ley 1392 de 2010. Fecha de consulta: 18 de noviembre de 2014. Disponible en: http://www.ins.gov.co/normatividad/Leyes/LEY%201392%20DE%202010.pdf.
Ministerio de Salud de Colombia. Resolución 412 de 2000. Fecha de consulta: 18 de noviembre de 2016. Disponible en: http://www.saludcolombia.com/actual/htmlnormas/Res412_00.htm
Ministerio de Salud de Colombia. Resolución 3384 de 2000. Fecha de consulta: 13 de marzo de 2017. Disponible en: https://www.minsalud.gov.co/Normatividad_Nuevo/Resoluci%C3%93N%203384%20DE%202000.pdf
Kelly N, Makarem DC, Wasserstein MP. Screening of newborns for disorders with high benefit-risk ratios should be mandatory. J Law Med Ethics. 2016;44:231-40. https://doi.org/10.1177/1073110516654133
Botkin JR, Goldenberg AJ, Rothwell E, Anderson RA, Lewis MH. Retention and research use of residual newborn screening bloodspots. Pediatrics. 2013;131:120-7. https://doi.org/10.1542/peds.2012-0852
Congreso de la República de Colombia. Ley 1805 de 2016. Fecha de consulta: 6 de agosto de 2020. Disponible en: https://dapre.presidencia.gov.co/normativa/normativa/LEY%201805%20DEL%2004%20DE%20AGOSTO%20DE%202016.pdf
Beauchamp T, Childress JF. El respeto de la autonomía. Principios de bioética médica. New York: Oxford University Press; 2009. p. 113-78.
Almond B. Genetic profiling of newborns: Ethical and social issues. Nat Rev Genet. 2006;7:67-71. https://doi.org/10.1038/nrg1745
UNESCO. Declaración Universal sobre el Genoma Humano y los Derechos Humanos 1997. Fecha de consulta: 9 de febrero de 2015. Disponible en:
http://unesdoc.unesco.org/images/0012/001229/122990so.pdf
Ministerio de Salud de Colombia. Resolución 1995 de 1999. Fecha de consulta: 6 de agosto de 2020. Disponible en: https://www.minsalud.gov.co/Normatividad_Nuevo/RESOLUCI%C3%93N%201995%20DE%201999.pdf
Duquette D, Langbo C, Bach J, Kleyn M. Michigan BioTrust for Health: public support for using residual dried blood spot samples for health research. Public Health Genomics. 2012;15:146-55. https://doi.org/10.1159/000336565
UNESCO. Declaración Internacional sobre los datos genéticos humanos, 2003. Fecha de consulta: 6 de agosto de 2020. Disponible en: http://www.leloir.org.ar/cbfil/wp-content/uploads/sites/57/2016/07/UNESCO_Declaraci%C3%B3n-Internacional-sobre-Datos-Gen%C3%A9ticos-Humanos_2003.pdf
Ministerio de Salud de Colombia. Resolución 8430 de 1993. Fecha de consulta: 6 de agosto de 2020. Disponible en: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/RESOLUCION-8430-DE-1993.PDF
Sánchez C. El derecho a la protección de la intimidad de los datos genéticos. Rev Latinoam Bioet. 2002:104-15.
Pelayo A. Bioética, bioderecho y biopolítica. Una aproximación desde España. Criterio Jurídico Garantista. 2012:4:12-35. https://doi.org/10.26564/21453381.398
Some similar items:
- Álvaro Ruiz, Right to die with dignity? , Biomedica: Vol. 28 No. 2 (2008)
- Neivis Marrero, Amarilys Frómeta, Remigio Coto, Layda Villegas, Measurements of TSH, T4 and Phe in samples of umbilical cord blood on filter paper: impact on neonatal screening , Biomedica: Vol. 20 No. 1 (2000)
- Amarilys Frómeta, Magdalena Barrios, Odalis Hernández, Elena Lugo, Arlenys Baloy, lnfluence of some factors associated with pregnancy and delivery on thyroid stimulating hormone (TSH) concentrations in umbilical cord sera , Biomedica: Vol. 21 No. 3 (2001)
- Ernesto C. González, Libertad Diaz, Amarilys Frómeta, Darlenis Herrera, Adonis Montenegro, Quantitative assay to determine the hydrolytic activity of biotinidase , Biomedica: Vol. 21 No. 4 (2001)
- Eneida Torres, Arlenys Baloy, Amarilys Frómeta, Lianny Fernández, Phenylalanine and total galactose measurement from dried blood spotted on filter paper application to newborn screening , Biomedica: Vol. 22 No. 1 (2002)
- Julio Cesar Mateus, María Teresa Varela, Diana María Caicedo, Nhora Lucía Arias, Cruz Deisy Jaramillo, Liliana Cristina Morales, Gloria Inés Palma, Does Resolution 8430 of 1993 respond to the current needs of ethics in health research with human beings in Colombia? , Biomedica: Vol. 39 No. 3 (2019)
- Lorena Peñaloza, Catalina Forero, Camila Céspedes, Characterization of patients diagnosed with congenital hypothyroidism at the Hospital Universitario San Ignacio between 2001 and 2017 , Biomedica: Vol. 40 No. 3 (2020)
- Jorge Alberto Restrepo , Luis Carlos Domínguez , Marcelo García-Diéguez , Learning climate and work engagement in clinical residents: The relationship with human self-determination , Biomedica: Vol. 42 No. 1 (2022)
- Jaime E. Bernal, Martha Lucía Tamayo , Ignacio Briceño , Escilda Benavides , Newborn screening in Colombia: The experience of a private program in Bogotá , Biomedica: Vol. 44 No. 1 (2024)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























