Performance of rapid IgM-IgG combined antibody tests in the occupational surveillance of COVID-19 in Colombian enterprises
Abstract
Introduction: Rapid IgM-IgG combined antibody tests can play an important role in the COVID-19 surveillance by supporting the diagnosis of infection, assessing the immune response, and verifying the progress towards herd immunity.
Objective: To evaluate the performance of rapid IgM-IgG combined antibody tests in COVID-19 occupational surveillance in a group of Colombian enterprises.
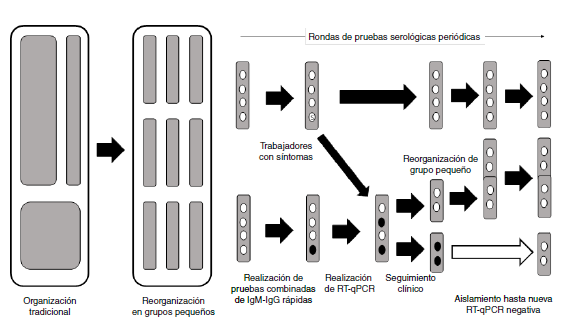
Materials and methods: We used the occupational surveillance data from companies that had performed periodic serological tests on all personnel from the end of April to the beginning of July, 2020. Workers were organized in small groups (“social bubbles”) to prevent outbreaks and optimize surveillance. The sensitivity was estimated as if the sampling had a prospective design. We describe here the changes in serological testing through periodic rounds.
Results: Data were obtained from 4,740 workers, of whom only 23 were symptomatic showing changes from IgM(-)/IgG(-) to IgM(+) and then to IgM(+)/IgG(+) and IgG(+). The sensitivity was 40.94% for IgM(+) and 47.95% for IgM(+)/IgG(+). This implies that a little less than half of the cases can be detected.
Conclusion: Antibody rapid tests have a role in the diagnostic process of infection and they must be evaluated taking into account the moment of the epidemic, the type of test purchased, and the populations at risk since their results depend on the number of infections and cases. In the context of a health crisis, they can be optimized by organizing workers into “social bubbles”
Downloads
References
Bambra C, Riordan R, Ford J, Matthews F. The COVID-19 pandemic and health inequalities. J Epidemiol Community Health. 2020:74:964-8. https://doi.org/10.1136/jech-2020-214401
Douglas M, Katikireddi SV, Taulbut M, McKee M, McCartney G. Mitigating the wider health effects of covid-19 pandemic response. Br Med J .2020;369:m1557. https://doi.org/10.1136/bmj.m1557
McKee M, Stuckler D. If the world fails to protect the economy, COVID-19 will damage health ot just now but also in the future. Nature Med. 2020;26:640-2. https://doi.org/10.1038/s41591-020-0863-y
Leclerc QJ, Fuller NM, Knight LE, CMMID COVID-19 Working Group, Funk S, Knight GM. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res. 2020;5:83. https://doi.org/10.12688/wellcomeopenres.15889.2
Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet. 2020;396:535-44. https://doi.org/10.1016/S0140-6736(20)31483-5
Manrique-Hernández EF, Moreno-Montoya J, Hurtado-Ortiz A, Prieto-Alvarado FE, Idrovo AJ. Desempeño del sistema de vigilancia colombiano durante la pandemia de COVID-19: evaluación rápida de los primeros 50 días. Biomédica 2020;40(Supl.2):96-103. https://doi.org/10.7705/biomedica.5582
Coronel-Sánchez VH, Álvarez-Pabón Y, Esteban LY, Vargas-Valero Ó, Omaña JJ, Idrovo AJ. Lay-based morbidity profiles of sugar cane workers: Testing a new method using free lists. J Prim Prev. 2020;41:39-49 https://doi.org/10.1007/s10935-019-00575-y
Shah D. Healthy worker effect phenomenon. Indian J Occup Environ Med. 2009;13:77-9. https://doi.org/10.4103/0019-5278.55123
Block P, Hoffman M, Raabe IJ, Dowd JB, Rahal C, Kashyap R, Mills MC. Social networkbased distancing strategies to flatten the COVID-19 curve in a post-lockdown world. Nat Hum Behav. 2020;4:588-96. https://doi.org/10.1038/s41562-020-0898-6
Estrada-Orozco K, Robayo A, Arévalo A, Zabaleta G, Mercado-Reyes M. Validación secundaria y verificación del desempeño de la prueba rápida “COVID-19 IgG/IgM Rapid Test Device”. Bogotá: Instituto Nacional de Salud, Instituto de Evaluación Tecnológica en Salud; 2020.
Ministerio de Salud y Protección Social. Lineamientos para el uso de pruebas moleculares RT-PCR, pruebas de antígeno y pruebas serológicas para SARS-COV-2 (COVID-19) en Colombia. Bogotá: Ministerio de Salud y Protección Social; 2020.
Orozco LC, Camargo DM. Evaluación de tecnologías diagnósticas y tipos de muestreos. Biomédica. 1997;17:321-4. https://doi.org/10.7705/biomedica.v17i4.964
Kraemer HC. Evaluating medical tests. Objective and quantitative guidelines. Newbury Park: Sage Publications; 1992.
dos Santos WG. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed Pharmacother. 2020;129:110493. https://doi.org/10.1016/j.biopha.2020.110493
Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249-51. https://doi.org/10.1001/jama.2020.8259
Bastos ML, Tavaziva G, Abidi SK, Campbell JR, Haraoui LP, Johnston JC, et al. Diagnostic accuracy of serological tests for covid-19: Systematic review and meta-analysis. Br Med J. 2020;370:m2516. https://doi.org/10.1136/bmj.m2516
Mahé C, Gaffikin L. Screening test accuracy studies: How valid are our conclusions? Application to visual inspection methods for cervical screening. Cancer Causes Control. 2005;16:657-66. https://doi.org/10.1007/s10552-005-0296-4
Kosack CS, Page AL, Klatser PR. A guide to aid the selection of diagnostic tests. Bull World Health Organ. 2017;95:639-45. https://doi.org/10.2471/BLT.16.187468
Larremore DB, Wilder B, Lester E, Shehata S, Burke JM, Hay JA, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. medRxiv. 2020. https://doi.org/10.1101/2020.06.22.20136309
Show J, Failing the coronavirus-testing test. Harvard Magazine, 2020. Fecha de consulta: 17 de julio de 2020. Disponible en: https://harvardmagazine.com/2020/08/covid-19-test-forpublic-health
Dorfman R. The detection of defective members of large populations. Ann Mat Stat. 1943;14:436-40.
Nguyen NT, Bish EK, Aprahamian H. Sequential prevalence estimation with pooling and continuous test outcomes. Stat Med. 2018;37:2391-426. https://doi.org/10.1002/sim.7657
Procuraduría General de la Nación. 15.053 resultados de pruebas para COVID-19 no han sido entregados: Bogotá: Procuraduría General de la Nación; 2020. Fecha de consulta: 28 de mayo de 2020. Disponible en: https://www.procuraduria.gov.co/portal/15053-resultadosde-pruebas-para-covid-19-no-han-sido-entregados-Procuraduria.news
Guenther T, Czech-Sioli M, Indenbirken D, Robitailles A, Tenhaken P, Exner M, et al. Investigation of a superspreading event preceding the largest meat processing plantrelated SARS-Coronavirus 2 outbreak in Germany. Fecha de consulta: 17 de julio de 2020. Disponible en: https://doi.org/10.2139/ssrn.3654517
Randolph HE, Barreiro LB. Herd immunity: Understanding COVID-19. Immunity. 2020;52:737-41. https://doi.org/10.1016/j.immuni.2020.04.012
Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860-8. https://doi.org/10.1126/science.abb5793
Tang YW, Schmitz JE, Persing DH, Stratton CW. The laboratory diagnosis of COVID-19 infection: Current issues and challenges. J Clin Microbiol. 2020;58:e00512-20. https://doi.org/10.1128/JCM.00512-20
Some similar items:
- Alvaro Javier Idrovo, José Moreno-Montoya, RT-qPCR diagnosis in state public health laboratories as determinants of performance of public health surveillance during the pandemic , Biomedica: Vol. 41 No. 2 (2021)
- Alexandra Hurtado-Ortiz, José Moreno-Montoya , Franklyn E. Prieto-Alvarado , Álvaro J. Idrovo, Benchmarking of public health surveillance of COVID-19 in Colombia: First semester , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Jose William Martínez, Juan Camilo Martínez , Diego Alejandro Rincón, Diego Alejandro Salazar, Juan Daniel Castrillón, María del Pilar Gómez, Oscar Felipe Suárez , Juan Pablo Vélez, Ángela María Valencia, Sandra Gómez, Ángel María Rincón , Álvaro J. Idrovo, José Moreno-Montoya, Franklyn E. Prieto-Alvarado, Alexandra Hurtado-Ortiz, Benchmarking of public health surveillance of COVID-19 in Colombia: First semester , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Edgar F. Manrique-Hernández, José Moreno-Montoya, Alexandra Hurtado-Ortiz, Franklyn E. Prieto-Alvarado, Álvaro J. Idrovo, Performance of the Colombian surveillance system during the COVID-19 pandemic: A rapid evaluation of the first 50 days , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Jeadran Malagón-Rojas, Claudia Gómez-Rendón , Eliana L. Parra , Julia Almentero , Ruth Palma , Ronald López , Yesith Guillermo Toloza-Pérez , Vivian Rubio , Juan Felipe Bedoya , Fernando López-Díaz , Carlos Franco-Muñoz , Jhonnatan Reales-González , Marcela Mercado-Reyes , SARS-CoV-2 and RT-PCR in asymptomatic patients: Results of a cohort of workers at El Dorado International Airport in Bogotá, 2020 , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Luis Mauricio Figueroa, Telehealth in Colombia, challenges associated with COVID-19 , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Zulma M. Cucunubá, Latin American scientific research prorities for COVID-19 prevention and control , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Wbeimar Aguilar-Jiménez, Wildeman Zapata, María Teresa Rugeles, Differential expression of human beta defensins in placenta and detection of allelic variants in the DEFB1 gene from HIV-1 positive mothers , Biomedica: Vol. 31 No. 1 (2011)
- Jairo Lizarazo, Paradoxical appearance of encephalic tuberculomas during treatment for tuberculosis in immunocompetent patients. , Biomedica: Vol. 24 (2004): Suplemento 1
- Judy Natalia Jiménez, Carlos Enrique Muskus, Iván Darío Vélez, Genetic diversity of Plasmodium falciparum and its implications in the epidemiology of malaria. , Biomedica: Vol. 25 No. 4 (2005)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























