Analysis of SOD1 and C9orf72 mutations in patients with amyotrophic lateral sclerosis in Antioquia, Colombia
Abstract
Introduction: Amyotrophic lateral sclerosis is a neurodegenerative disease with a possible multifactorial origin characterized by the progressive degeneration of motor neurons. There is a relatively high prevalence of this disease in Antioquia; however, there is no published genetic study to date in Colombia. Despite its unknown etiopathogenesis, more genetic risk factors possibly involved in the development of this disease are constantly found.
Objetives: To evaluate G93A and D90A mutations in SOD1 gene and a short tandem repeat in C9orf72 within a cohort of amyotrophic lateral sclerosis patients from Antioquia, Colombia.
Materials y methods: Thirty-four patients previously diagnosed with amyotrophic lateral sclerosis were included in the study. Peripheral blood samples were used for DNA extraction and genotyping.
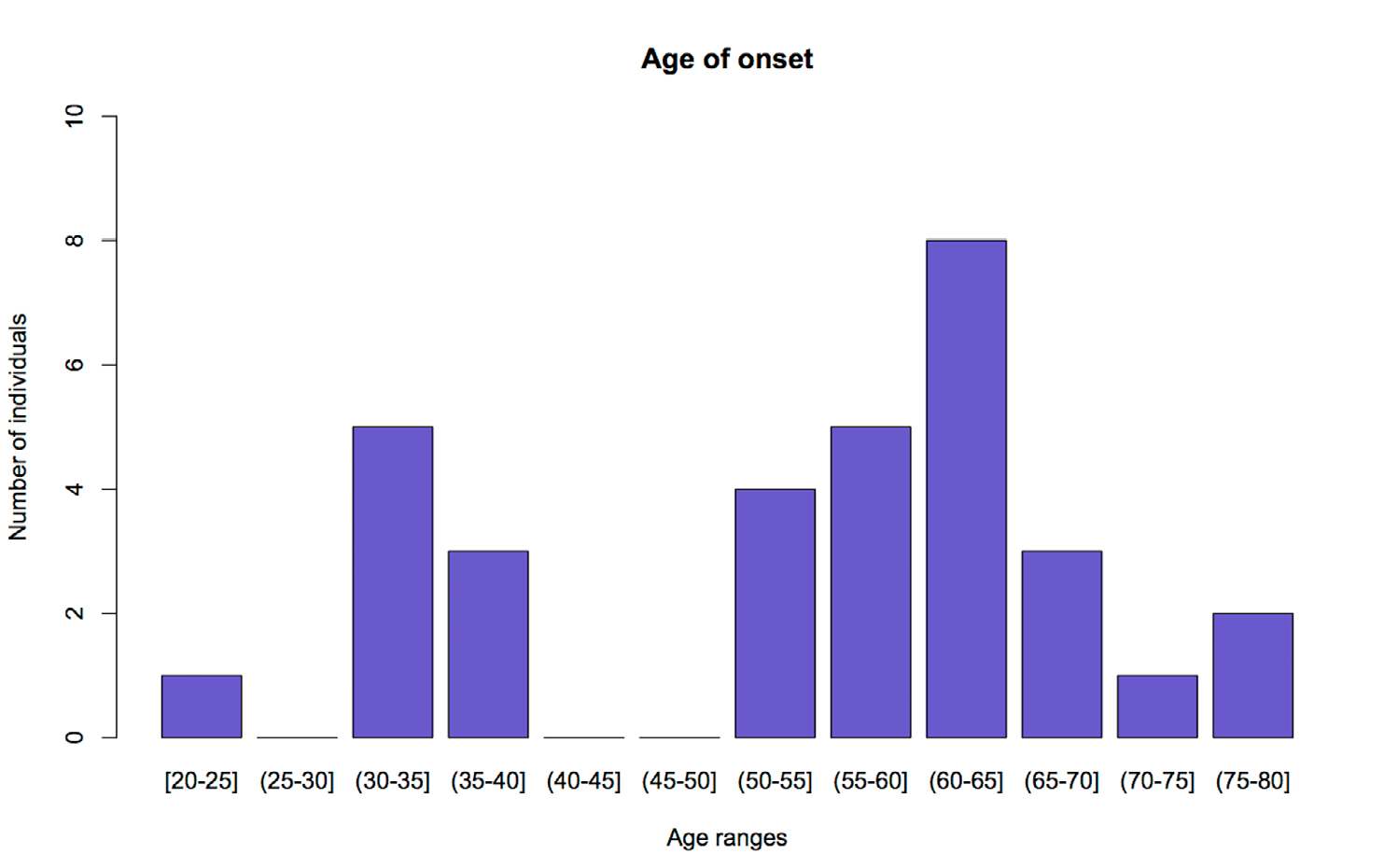
Results: No mutations were found in SOD1 (G93A and D90A) in any of the patients, while C9orf72 exhibited an allele with a statistically significant high prevalence in the study sample (8 hexanucleotide repeats of CAGCAG).
Conclusions: These results suggest an association between this short tandem repeat (STR) in C9orf72 and the presence of amyotrophic lateral sclerosis in the studied population. However, this association should be established in a larger sample size and with controls from the same population. In addition, there also seems to be a genetic anticipation effect for the disease regarding this locus, since patients with this genotype present an earlier onset.
Downloads
References
Yamashita S, Ando Y. Genotype-phenotype relationship in hereditary amyotrophic lateral sclerosis. Transl Neurodegener. 2015;4:13. https://doi.org/10.1186/s40035-015-0036-y
Turner MR, Verstraete E. What does imaging reveal about the pathology of amyotrophic lateral sclerosis? Curr Neurol Neurosci Rep. 2015;15:45. https://doi.org/10.1007/s11910-015-0569-6
Hamosh A. Online Mendelian Inheritance in Man (OMIM), a knowledge base of human genes and genetic disorders. Nucleic Acids Res. 2004;33:D514-7. https://doi.org/10.1093/nar/gki033
Yu W, Gwinn M, Clyne M, Yesupriya A, Khoury MJ. A navigator for human genome epidemiology. Nat Genet. 2008;40:124-5. https://doi.org/10.1038/ng0208-124
Rohrer JD, Isaacs AM, Mizielinska S, Mead S, Lashley T, Wray S, et al. C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol. 2015;14:291-301. https://doi.org/10.1016/S1474-4422(14)70233-9
Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 2009;4:3. https://doi.org/10.1186/1750-1172-4-3
Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). BBA -Mol Basis of Dis. 2015;1852:679-84. https://doi.org/10.1016/j.bbadis.2014.08.010
De Jong SW, Huisman MHB, Sutedja NA, van der Kooi AJ, de Visser M, Schelhaas HJ, et al. Smoking, alcohol consumption, and the risk of amyotrophic lateral sclerosis: A populationbased study. Am J Epidemiol. 2012;176:233-9. https://doi.org/ 10.1093/aje/kws015
Saez-Atienzar S, Bandres-Ciga S, Langston RG, Kim JJ, Choi SW, Reynolds RH, et al. Genetic analysis of amyotrophic lateral sclerosis identifies contributing pathways and cell types. Sci Adv. 2021;7:eabd9036. https://doi.org/10.1126/sciadv.abd9036
Özoğuz A, Uyan Ö, Birdal G, Iskender C, Kartal E, Lahut S, et al. The distinct genetic pattern of ALS in Turkey and novel mutations. Neurobiol Aging. 2015;36:1764.e9-1764.e18. https://doi.org/10.1016/j.neurobiolaging.2014.12.032
Bertolin C, D’Ascenzo C, Querin G, Gaiani A, Boaretto F, Salvoro C, et al. Improving the knowledge of amyotrophic lateral sclerosis genetics: Novel SOD1 and FUS variants. Neurob Aging. 2014;35:1212. http://doi.org/10.1016/j.neurobiolaging.2013.10.093
Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: A population-based cohort study. Lancet Neurol. 2012;11:232-40. https://doi.org/10.1016/S1474-4422(12)70014-5
Ugolino J, Ji YJ, Conchina K, Chu J, Nirujogi RS, Pandey A, et al. Loss of C9orf72 enhances autophagic activity via deregulated mTOR and TFEB signaling. PLOS Genet. 2016;12:e1006443. https://doi.org/10.1371/journal.pgen.1006443
Isobe T, Tooi N, Nakatsuji N, Aiba K. Amyotrophic lateral sclerosis models derived from human embryonic stem cells with different superoxide dismutase 1 mutations exhibit differential drug responses. Stem Cell Res. 2015;15:459-68. https://doi.org/10.1016/j.scr.2015.09.006
Mancuso R, Navarro X. Amyotrophic lateral sclerosis: Current perspectives from basic research to the clinic. Prog Neurobiol. 2015;133:1-26. https://doi.org/10.1016/j.pneurobio.2015.07.004
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9orf72 is the cause of chromosome 9p21-Linked ALSFTD. Neuron. 2011;72:257-68. https://doi.org/10.1016/j.neuron.2011.09.010
Ng ASL, Tan EK. Intermediate C9orf72 alleles in neurological disorders: Does size really matter? J Med Genet. 2017;54:591-7. https://doi.org/10.1136/jmedgenet-2017-104752
Ospina ML, Prieto FE, Pachecho O, Quijano H, Lozano N, Gomez S, et al. Boletín epidemiológico semana 5. 2019. Fecha de consulta: 4 de febrero de 2021. Disponible en: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2019%20Bolet%C3%ADn%20epidemiol%C3%B3gico%20semana%205.pdf
Zapata-Zapata C, Franco-Dáger E, Solano-Atehortúa J, Ahunca-Velásquez L. Esclerosis lateral amiotrófica: actualización. IATREIA. 2016;29:194-205. https://doi.org/10.17533/udea.iatreia.v29n2a08
Zapata-Zapata CH, Dáger EF, Aguirre-Acevedo DC, de Carvalho M, Solano-Atehortua J. Prevalence, incidence, and clinical-epidemiological characterization of amyotrophic lateral sclerosis in Antioquia: Colombia. Neuroepidemiology. 2020;54:251-8. https://doi.org/ 10.1159/000504549
Nikali K, Vanegas JJ, Burley MW, Martínez J, López LM, Bedoya G, et al. Extensive founder effect for distal renal tubular acidosis (dRTA) with sensorineural deafness in an isolated South American population. Am J Med Genet. 2008;146A:2709-12. https://doi.org/ 10.1002/ajmg.a.32495
Carvajal-Carmona LG, Soto ID, Pineda N, Ortiz-Barrientos D, Duque C, Ospina-Duque J, et al. Strong amerind/white sex bias and a possible sephardic contribution among the founders of a population in Northwest Colombia. Am J Hum Genet. 2000;67:1287-95. https://doi.org/10.1016/s0002-9297(07)62956-5
Arcos-Burgos M, Muenke M. Genetics of population isolates. Clin Genet. 2002;61:233-47. https://doi.org/ 10.1034/j.1399-0004.2002.610401.x
De Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119:497-503. https://doi.org/ 10.1016/j.clinph.2007.09.143
DeJesús-Hernández M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9orf72 causes chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245-56. https://doi.org/10.1016/j.neuron.2011.09.011
Martín Andrés A. Entry Fisher’s exact and Barnard’s tests. In: Kotz S, Johnson NL, Read CB, editors. Encyclopedia of Statistical Sciences. New York, NY: Wiley-Interscience; 1998. p. 250-8. https://doi.org/10.1002/0471667196.ess0642.pub2
Rousset F. genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour. 2008;8:103-6. https://doi.org/10.1111/j.1471-8286.2007.01931.x
Raymond M, Rousset F. GENEPOP (Version 1.2): Population genetics software for exact tests and ecumenicism. J Hered. 1995;86:248-9. https://doi.org/10.1093/oxfordjournals.jhered.a111573
Couthouis J, Raphael AR, Daneshjou R, Gitler AD. Targeted exon capture and sequencing in sporadic amyotrophic lateral sclerosis. PLoS Genet. 2014;10:e1004704. https://doi.org/10.1371/journal.pgen.1004704
Marin B, Boumédiene F, Logroscino G, Couratier P, Babron MC, Leutenegger AL, et al. Variation in worldwide incidence of amyotrophic lateral sclerosis: A meta-analysis. Int J Epidemiol. 2017;46:57-74. https://doi.org/10.1093/ije/dyw061
Chiò A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al. Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler. 2009;10:310-23. https://doi.org/10.3109/17482960802566824
Logroscino G, Piccininni M. Amyotrophic lateral sclerosis descriptive epidemiology: The Origin of geographic difference. Neuroepidemiology. 2019;52:93-103. https://doi.org/10.1159/000493386
Carvajal-Carmona LG, Ophoff R, Service S, Hartiala J, Molina J, León P, et al. Genetic demography of Antioquia (Colombia) and the Central Valley of Costa Rica. Hum Genet. 2003;112:534-41. https://doi.org/10.1007/s00439-002-0899-8
Chiurazzi P, Oostra BA. Expanding Mutations/Genetic Anticipation. Encyclopedia of Life Sciences. Chichester, UK: John Wiley & Sons, Ltd.; 2006. https://doi.org/10.1038/npg.els.0001463
Suzuki N, Nishiyama A, Warita H, Aoki M. Genetics of amyotrophic lateral sclerosis: Seeking therapeutic targets in the era of gene therapy. J Hum Genet. 2022. https://doi.org/10.1038/s10038-022-01055-8
Byrne S, Heverin M, Elamin M, Walsh C, Hardiman O. Intermediate repeat expansion length in C9orf72 may be pathological in amyotrophic lateral sclerosis. Amyotrop Lateral Scler Frontotemporal Degener. 2014;15:148-50. https://doi.org/10.3109/21678421.2013.838586
Yang Q, Jiao B, Shen L. The development of C9orf72-related amyotrophic lateral sclerosis and frontotemporal dementia disorders. Front Genet. 2020. https://doi.org/10.3389/fgene.2020.562758
Mitteroecker P, Cheverud JM, Pavlicev M. Multivariate analysis of genotype–phenotype association. Genet. 2016;202:1345-63. https://doi.org/10.1534/genetics.115.181339
Namipashaki A, Razaghi-Moghadam Z, Ansari-Pour N. The essentiality of reporting hardyweinberg equilibrium calculations in population-based genetic association studies. Cell J. 2015;17:187-92. https://doi.org/10.22074/cellj.2016.3711
Hedrick PW. Genetics of populations. 4th edition. Sudbury, MA: Jones and Bartlett Publishers; 2011. p. 65-109.
Nakamura R, Misawa K, Tohnai G, Nakatochi M, Furuhashi S, Atsuta N, et al. A multi-ethnic meta-analysis identifies novel genes, including ACSL5, associated with amyotrophic lateral sclerosis. Commun Biol. 2020;3:526. https://doi.org/10.1038/s42003-020-01251-2
Mesaros M, Lenz S, Lim W, Brown J, Drury L, Roggenbuck J. Investigating the genetic profile of the amyotrophic lateral sclerosis/frontotemporal dementia (ALS-FTD) continuum in patients of diverse race, ethnicity and ancestry. Genes (Basel). 2021;13:76. https://doi.org/10.3390/genes13010076
Štětkářová I, Ehler E. Diagnostics of amyotrophic lateral sclerosis: Up to date. Diagnostics (Basel). 2021;11:231. https://doi.org/10.3390/diagnostics11020231
Huang F, Zhu Y, Hsiao-Nakamoto J, Tang X, Dugas JC, Moscovitch-Lopatin M, et al. Longitudinal biomarkers in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2020;7:1103-16. https://doi.org/10.1002/acn3.51078
Álvarez-Fernández A, Bernal MJ, Fradejas I, Martín Ramírez A, Md Yusuf NA, Lanza M, et al. KASP: A genotyping method to rapid identification of resistance in Plasmodium falciparum. Malar J. 2021;20:16. https://doi.org/10.1186/s12936-020-03544-7
Some similar items:
- Martha Lucía Serrano, Juan José Yunis, Identification of three new mutations in the RB1 gene in patients with sporadic retinoblastoma in Colombia , Biomedica: Vol. 33 No. 1 (2013)
- María Carolina Sanabria, Gerardo Muñoz, Clara Inés Vargas, Mutations in the BRCA1 gene (185delAG and 5382insC) are not present in any of the 30 breast cancer patients analyzed from eastern Colombia , Biomedica: Vol. 29 No. 1 (2009)
- Brian Alejandro Suárez, Claudia Liliana Cuervo, Concepción Judith Puerta, The intergenic region of the histone h2a gene supports two major lineages of Trypanosoma rangeli , Biomedica: Vol. 27 No. 3 (2007)
- Patricia Escandón, Popchai Ngamskulrungroj, Wieland Meyer, Elizabeth Castañeda, In vitro mating of Colombian isolates of the Cryptococcus neoformans species complex , Biomedica: Vol. 27 No. 2 (2007)
- Winston Rojas, Maria Antonieta Caro, Juan Guillermo Lopera, Omar Triana, Juan Carlos Dib, Gabriel Bedoya, Analysis of polymorphisms in the trypanothione reductase and cruzipain genes in Colombian strains of Trypanosoma cruzi , Biomedica: Vol. 27 No. 1esp (2007): Enfermedad de Chagas
- Juan Carlos Herrera, Luis Fernando Isaza, José Luis Ramírez, Gonzalo Vásquez, Carlos Mario Muñetón, Detection of chromosome 17 aneuplody and TP53 gene deletion in a broad variety of solid tumors by dual-color fluorescence in situ hybridization (FISH) , Biomedica: Vol. 30 No. 3 (2010)
- Andrea Gómez, Gustavo Salguero, Herbert García, Fabio Aristizábal, Oscar Gutiérrez, Luis Alberto Angel, Jorge Padrón, Carlos Martínez, Humberto Martínez, Omar Malaver, Rosa Barvo, Alejandro Giraldo, Detection mutations in the DNA mismatch repair genes of hMLH1 and hMSH2 genes in Colombian families with suspicion of hereditary non-polyposis colorectal carcinoma (Lynch syndrome). , Biomedica: Vol. 25 No. 3 (2005)
- Carlos Andrés Ossa, Gustavo Molina, Alicia María Cock-Rada, Li-Fraumeni syndrome , Biomedica: Vol. 36 No. 2 (2016)
- Claudia Patricia Acosta, Andrés Javier Quiroga, Carlos H. Sierra, Alba Alicia Trespalacios, Frequency of Helicobacter pylori nitroreductase RdxA mutations for metronidazole activation in a population in the Cauca Department, Colombia , Biomedica: Vol. 37 No. 2 (2017)
- Jennyfer Benavides , Jonh Suárez, Ana Estrada, Mábel Bohórquez, Carolina Ramírez, Justo Olaya , Yesid Sánchez , Gilbert Mateus , Luis Carvajal , María Magdalena Echeverry , Breast cancer in six families from Tolima and Huila: BRCA1 3450del4 mutation , Biomedica: Vol. 40 No. 1 (2020)
Copyright (c) 2022 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























