Phenotypic characterization of retinitis pigmentosa associated with deafness
Abstract
Introduction: There are several syndromes that associate retinitis pigmentosa with deafness or hearing loss. The most frequent is Usher syndrome, a genetic disorder of autosomal recessive inheritance, which, in some cases, is accompanied by vestibular dysfunction. However, there are cases of families that despite having retinitis pigmentosa associated with deafness, cannot be classified as Usher or other syndromes due to additional findings.
Objective: To reassess the phenotypes of 103 families previously diagnosed as possible Usher syndrome and/or retinitis pigmentosa associated with deafness.
Materials and methods: We conducted a descriptive and retrospective study by reviewing the medical records of 103 families with a probable clinical diagnosis of Usher syndrome and/or retinitis pigmentosa associated with deafness. Families whose clinical diagnosis did not correspond to the typical Usher syndrome were selected and evaluated ophthalmologically and audiologically. Demographic and clinical variables were analyzed.
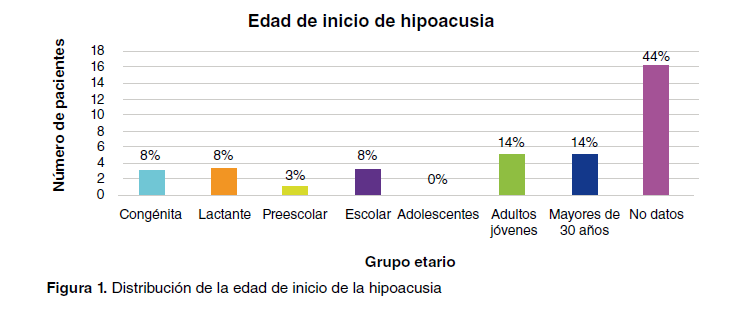
Results: We selected and then reevaluated 14 families and 55 individuals as they did not correspond to a clinical diagnosis of Usher syndrome; 13.6% of the families initially considered to have typical Usher syndrome were later diagnosed with retinitis pigmentosa associated with deafness, another ocular symptom associated with hearing loss, retinitis pigmentosa, or isolated hearing loss in the same family.
Conclusions: Family studies are essential in cases where the symptoms do not match the typical Usher’ syndrome. In the cases of retinitis pigmentosa associated with deafness, a correct clinical diagnosis allows for focusing on the molecular analyses to establish a differential diagnosis. The need for nomenclature guidelines on these atypical findings is relevant to aid physicians and researchers in the best approach to these cases.
Downloads
References
Malm E, Ponjavic V, Möller C, Kimberling WJ, Andréasson S. Phenotypes in defined genotypes including siblings with Usher syndrome. Ophthalmic Genet. 2011;32:65-74.
https://doi.org/10.3109/13816810.2010.536064
Kaplan HJ, Wang W, Dean DC. Restoration of cone photoreceptor function in retinitis pigmentosa. Transl Vis Sci Technol. 2017;6:5. 10.1167/tvst.6.5.5 https://doi.org/10.1167/tvst.6.5.5
Pakarinen L, Tuppurainen K, Laippala P, Mäntyjärvi M, Puhakka H. The ophthalmological course of Usher syndrome type III. Ophthalmic Lit. 1997;1:36. https://doi.org/10.1007/BF00130927
Rabin J, Houser B, Talbert C, Patel R. Measurement of dark adaptometry during ISCEV standard flash electroretinography. Doc Ophthalmol. 2017;135:195-208. https://doi.org/10.1007/s10633-017-9614-x
Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS ONE. 2012;7:e28936. https://doi.org/10.1371/journal.pone.0028936
Parmeggiani F, Sorrentino F, Ponzin D, Barbaro V, Ferrari S, Di Lorio E. Retinitis pigmentosa: Genes and disease mechanisms. Curr Genomics. 2011;12:238-49. https://doi.org/10.2174/138920211795860107
Berson EL, Rosner B, Simonoff E. Risk factors for genetic typing and detection in retinitis pigmentosa. Am J Ophthalmol. 1980;89:763-75. https://doi.org/10.1016/0002-9394(80)90163-4
Cummings C, Fredrickson J, Harker L. Otolaryngology: Head and neck surgery. 4th edition. St Louis: Wiley Online Library; 2005. p. 2086-99.
Boughman JA, Vernon M, Shaver KA. Usher syndrome: Definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983;36:595-603. https://doi.org/10.1016/0021-9681(83)90147-9
Cortés RA, Cenjor C, Ayuso C. Síndrome de Usher: aspectos clínicos, diagnósticos y terapéuticos. Tesis. Madrid: Universidad Autónoma de Madrid; 2004.
Tamayo ML, Bernal JE, Tamayo GE, Frías JL, Alvira G, Vergara O, et al. Usher syndrome: Results of a screening program in Colombia. Clin Genet. 2008;40:304-11. https://doi.org/10.1111/j.1399-0004.1991.tb03100.x
Leal GL, Moyano NG, Tamayo ML. Definición de subtipos del síndrome de Usher en población colombiana. Medicina (Mex). 2010;32:285-94.
Tamayo ML, Bernal JE, Tamayo GE, Frías JL. Study of the etiology of deafness in an institutionalized population in Colombia. Am J Med Genet. 1992;44:405-8. https://doi.org/10.1002/ajmg.1320440403
Khan KN, El-Asrag ME, Ku CA, Holder GE, McKibbin M, Arno G, et al. Specific alleles of CLN7 / MFSD8, a protein that localizes to photoreceptor synaptic terminals, cause a spectrum of nonsyndromic retinal dystrophy. Investig Opthalmology Vis Sci. 2017;58:2906. https://doi.org/10.1167/iovs.16-20608
Neuhaus C, Eisenberger T, Decker C, Nagl S, Blank C, Pfister M, et al. Next-generation sequencing reveals the mutational landscape of clinically diagnosed Usher syndrome: Copy number variations, phenocopies, a predominant target for translational read-through, and PEX26 mutated in Heimler syndrome. Mol Genet Genomic Med. 2017;5:531-52. https://doi.org/10.1002/mgg3.312
Khateb S, Kowalewski B, Bedoni N, Damme M, Pollack N, Saada A, et al. A homozygous founder missense variant in arylsulfatase G abolishes its enzymatic activity causing atypical Usher syndrome in humans. Genet Med. 2018;20:1004-12. https://doi.org/10.1038/gim.2017.227
Trouillet A, Dubus E, Dégardin J, Estivalet A, Ivkovic I, Godefroy D, et al. Cone degeneration is triggered by the absence of USH1 proteins but prevented by antioxidant treatments. Sci Rep. 2018;8:1968. https://doi.org/10.1038/s41598-018-20171-0
Orejas J, Rico J. Hipoacusia: identificación e intervención precoces. Pediatría Integral. 2013;17:330-42.
Khateb S, Zelinger L, Mizrahi-Meissonnier L, Ayuso C, Koenekoop RK, Laxer U, et al. A homozygous nonsense CEP250 mutation combined with a heterozygous nonsense C2orf71 mutation is associated with atypical Usher syndrome. J Med Genet. 2014;51:460-9. https://doi.org/10.1136/jmedgenet-2014-102287
Namburi P, Ratnapriya R, Khateb S, Lazar CH, Kinarty Y, Obolensky A, et al. Bi-allelic truncating mutations in CEP78, encoding centrosomal protein 78, cause cone-rod degeneration with sensorineural hearing loss. Am J Hum Genet. 2016;99:777-84. https://doi.org/10.1016/j.ajhg.2016.07.010
Ren SM, Wu QH, Chen YB, Jiao ZH, Kong XD. Variation analysis of genes associated with Usher syndrome type 1 in 136 Chinese deafness families. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;56:236-41. https://doi.org/10.3760/cma.j.cn115330-20200407-00273
Lentz J, Keats B. Usher syndrome type II. In: Adam MP, Ardinger HH, Pagon RA, et al. (editors). GeneReviews®. Seattle: University of Washington; 2016. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK1341/
Lentz J, Keats BJ. Usher syndrome type I. In: Adam MP, Ardinger HH, Pagon RA, et al. (editors). GeneReviews®. Seattle: University of Washington; 2016. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK1265/
Delgado JJ, Grupo PrevInfad/PAPPS infancia y adolescencia. Detección precoz de la hipoacusia infantil. Rev Pediatr Aten Primaria. 2011;13:279-97.
Mathur P, Yang J. Usher syndrome: Hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta. 2015;1852:406-20. https://doi.org/10.1016/j.bbadis.2014.11.020
Eisenberger T, Slim R, Mansour A, Nauck M, Nürnberg G, Nürnberg P, et al. Targeted nextgeneration sequencing identifies a homozygous nonsense mutation in ABHD12, the gene underlying PHARC, in a family clinically diagnosed with Usher syndrome type 3. Orphanet J Rare Dis. 2012;7:59. https://doi.org/10.1186/1750-1172-7-59
Bauer C, Jenkins H. Otologic symptoms and syndromes. In: Flint PW, Haughey BH, Lund VJ, Niparko JK, Richardson MA, Robbins KT (editors). Cummings Otolaryngology Head & Neck Surgery. 6th edition. Philadelphia: Elsevier Saunders; 2015. p. 2100-15.
Hildebrand MS, Husein M, Smith RJ. Genetic sensorineural hearing loss. In: Flint PW, Haughey BH, Lund VJ, Niparko JK, Richardson MA, Robbins KT (editors). Cummings Otolaryngology Head & Neck Surgery. 6th edition. Philadelphia: Elsevier Saunders; 2015. p. 1887-1903.
López G, Gélvez NY, Tamayo M. Frecuencia de mutaciones en el gen de la usherina (USH2A) en 26 individuos colombianos con síndrome de Usher, tipo II. Biomédica. 2011;31:82-90. https://doi.org/10.7705/biomedica.v31i1.338
Ordóñez J, Tekin M. Deafness and myopia syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. (editors). GeneReviews®. Seattle: University of Washington; 2017. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK269029/
Shearer AE, Hildebrand MS, Smith RJ. Hereditary hearing loss and deafness overview. In: Adam MP, Ardinger HH, Pagon RA, et al. (editors). GeneReviews®. University of Washington, Seattle; 2017. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK1434/
Nagase Y, Kurata K, Hosono K, Suto K, Hikoya A, Nakanishi H, et al. Visual outcomes in Japanese patients with retinitis pigmentosa and Usher syndrome caused by USH2A mutations. Semin Ophthalmol. 2018;33:560-5. https://doi.org/10.1080/08820538.2017.1340487
Testa F, Melillo P, Bonnet C, Marcelli V, De Benedictis A, Colucci R, et al. Clinical presentation and disease course of Usher syndrome because of mutations in MYO7A or USH2A. Retina. 2017;37:1581-90. https://doi.org/10.1097/IAE.0000000000001389
Pater JA, Green J, O’Rielly DD, Griffin A, Squires J, Burt T, et al. Novel Usher syndrome pathogenic variants identified in cases with hearing and vision loss. BMC Med Genet. 2019;20:68. https://doi.org/10.1186/s12881-019-0777-z
Sun LW, Johnson RD, Langlo CS, Cooper RF, Razeen MM, Russillo MC, et al. Assessing photoreceptor structure in retinitis pigmentosa and Usher syndrome. Investig Opthalmology Vis Sci. 2016;57:2428. https://doi.org/10.1167/iovs.15-18246
Ayuso C, Millan JM. Retinitis pigmentosa and allied conditions today: A paradigm of translational research. Genome Med. 2010;2:34. https://doi.org/10.1186/gm155
Chassine T, Bocquet B, Daien V, Ávila-Fernández A, Ayuso C, Collin RW, et al. Autosomal recessive retinitis pigmentosa with RP1 mutations is associated with myopia. Br J Ophthalmol. 2015;99:1360-5. https://doi.org/10.1136/bjophthalmol-2014-306224
Zhang Y, Wildsoet CF. The RPE in myopia development. In: Klettner AK, Dithmar S (editors). Retinal pigment epithelium in health and disease. Cham: Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28384-1_7
Klettner AK, Dithmar S. Retinal pigment epithelium in health and disease. Cham: Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28384-1
Park H, Tan CC, Faulkner A, Jabbar SB, Schmid G, Abey J, et al. Retinal degeneration increases susceptibility to myopia in mice. Mol Vis. 2013;19:2068-79.
Gregory-Evans K, Pennesi ME, Weleber RG. Retinitis pigmentosa and allied disorders. In: Ryan SJ, Sadda SR, Schachat AP (editors). Retina. Los Angeles: Saunders; Elsevier; 2013. https://doi.org/10.1016/B978-1-4557-0737-9.00040-0
Sánchez-Tocino H, Díez-Montero C, Villanueva-Gómez A, Lobo-Valentín R, Montero-Moreno JA. Phenotypic high myopia in X-linked retinitis pigmentosa secondary to a novel mutation in the RPGR gene. Ophthalmic Genet. 2019;40:170-6. https://doi.org/10.1080/13816810.2019.1605385
Lu Y, Sun X. Retinitis pigmentosa sine pigmento masqueraded as myopia: A case report (CARE). Medicine (Baltimore). 2021;100:e24006. https://doi.org/10.1097/MD.0000000000024006
Dad S, Rendtorff ND, Tranebjaerg L, Grønskov K, Karstensen HG, Brox V, et al. Usher syndrome in Denmark: Mutation spectrum and some clinical observations. Mol Genet Genomic Med. 2016;4:527-39. https://doi.org/10.1002/mgg3.228
García-García G, Aparisi MJ, Jaijo T, Rodrigo R, León AM, Ávila-Fernández A, et al. Mutational screening of the USH2A gene in Spanish USH patients reveals 23 novel pathogenic mutations. Orphanet J Rare Dis. 2011;6:65. https://doi.org/10.1186/1750-1172-6-65
Bashir R, Fatima A, Naz S. A frameshift mutation in SANS results in atypical Usher syndrome. Clin Genet. 2010;78:601-3. https://doi.org/10.1111/j.1399-0004.2010.01500.x
Adam M, Ardinger H, Pagon R, Wallace S, Bean L, Stephens K, et al. Nonsyndromic hearing loss and deafness, DFNA3. In: GeneReviews®. Seattle: University of Washington; 1998. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK1536/
Hildebrand M, Thorne N, Bromhead C, Kahrizi K, Webster J, Fattahi Z, et al. Variable hearing impairment in a DFNB2 family with a novel MYO7A missense mutation. Clin Genet. 2010;77:563-71. https://doi.org/10.1111/j.1399-0004.2009.01344.x
Faundes V, Pardo RA, Castillo-Taucher S. Genética de la sordera congénita. Med Clínica. 2012;139:446-51. https://doi.org/10.1016/j.medcli.2012.02.014
Antenora A, Rinaldi C, Roca A, Pane C, Lieto M, Saccà F, et al. The multiple faces of spinocerebellar ataxia type 2. Ann Clin Transl Neurol. 2017;4:687-95. https://doi.org/10.1002/acn3.437
Rossi M, Pérez-Lloret S, Doldan L, Cerquetti D, Balej J, Millar-Vernetti P, et al. Autosomal dominant cerebellar ataxias: A systematic review of clinical features. Eur J Neurol. 2014;21:607-15. https://doi.org/10.1111/ene.12350
Giocondo F, Curcio G. Spinocerebellar ataxia: A critical review of cognitive and sociocognitive deficits. Int J Neurosci. 2018;128:182-91. https://doi.org/10.1080/00207454.2017.1377198
Nolen R, Hufnagel R, Friedman T, Turriff A, Brewer C, Zalewski C, et al. Atypical and ultrarare Usher syndrome: A review. Ophthalmic Genet. 2020;41:401-12. https://doi.org/10.1080/13816810.2020.1747090
Some similar items:
- Greizy López, Nancy Yaneth Gelvez, Martalucía Tamayo, Mutational frequencies in usherin (USH2A gene) in 26 Colombian individuals with Usher syndrome type II , Biomedica: Vol. 31 No. 1 (2011)
- Patricia Escandón, Popchai Ngamskulrungroj, Wieland Meyer, Elizabeth Castañeda, In vitro mating of Colombian isolates of the Cryptococcus neoformans species complex , Biomedica: Vol. 27 No. 2 (2007)
- Patricia Escandón, Elizabeth Quintero, Diana Granados, Sandra Huérfano, Alejandro Ruiz, Elizabeth Castañeda, Isolation of Cryptococcus gattii serotype B from detritus of Eucalyptus trees in Colombia. , Biomedica: Vol. 25 No. 3 (2005)
- Fredi Alexander Díaz, Ruth Aralí Martínez, Luis Angel Villar, Clinical criteria to diagnose dengue in its early stages. , Biomedica: Vol. 26 No. 1 (2006)
- Jorge A. Vega, Simón Villegas-Ospina, Wbeimar Aguilar-Jiménez, María T. Rugeles, Gabriel Bedoya, Wildeman Zapata, Haplotypes in CCR5-CCR2, CCL3 and CCL5 are associated with natural resistance to HIV-1 infection in a Colombian cohort , Biomedica: Vol. 37 No. 2 (2017)
- Luz Yaqueline Ladino, Johanna Galvis, Diana Yasnó, Adriana Ramírez, Orietta Ivonne Beltrán, A pathogenic homozygous variant of the BBS10 gene in a patient with Bardet Biedl syndrome , Biomedica: Vol. 38 No. 3 (2018)
- Carmen Lucía Curcio, Andrés Fernando Giraldo, Fernando Gómez, The healthy aging phenotype in older people in Manizales , Biomedica: Vol. 40 No. 1 (2020)
- Laura C. Álvarez-Acevedo, María C. Zuleta-González, Óscar M. Gómez-Guzmán, Álvaro L. Rúa-Giraldo, MSc PhD, M.D. PhD, Martha E. Urán-Jiménez, Myrtha Arango-Arteaga, MSc PhD, Manoel Marques Evangelista de Oliveira, María del P. Jiménez-Alzate, Phenotypic and genotypic characterization of Colombian clinical isolates of Sporothrix spp. , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- Jenny Andrea Sierra, Mónica Montaña, Karla Rugeles , María Teresa Sandoval , Wilson Sandoval , Karem Johanna Delgado, Jhon Jairo Abella, Hearing health characterization and exposure to noise in the population aged 18 to 64 in Bogotá D.C., years 2014-2018 , Biomedica: Vol. 44 No. 2 (2024): Publicación anticipada, junio
Funding data
-
Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS)
Grant numbers Cod.120372453645. Contrato 656-2015
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























