Frequency of strongyloidiasis and associated factors: Analysis of 13 years of laboratory results in a tertiary referral hospital in Honduras, 2010-2022
Abstract
Introduction. The frequency of detected strongyloidiasis is affected by the selected laboratory method in the studied population. Considering that Honduras has few
community-based studies, the analysis of the laboratory record data can provide information helping to understand this parasitosis.
Objective. To estimate the frequency and to identify the factors associated with strongyloidiasis, analyzing the laboratory records of the Servicio de Parasitología at
Hospital Escuela in Tegucigalpa (Honduras) between 2010 and 2022.
Materials and methods. We carried out a descriptive, cross-sectional, analytical study. The laboratory diagnosis consisted of stool samples’ examination by direct smear and modified Baermann technique. We estimated frequencies and percentages. The statistical association was calculated with prevalence ratios and a 95% confidence interval. Software R, version 4.2.0, and epiR package, version 2.0.46, were used to perform the analysis.
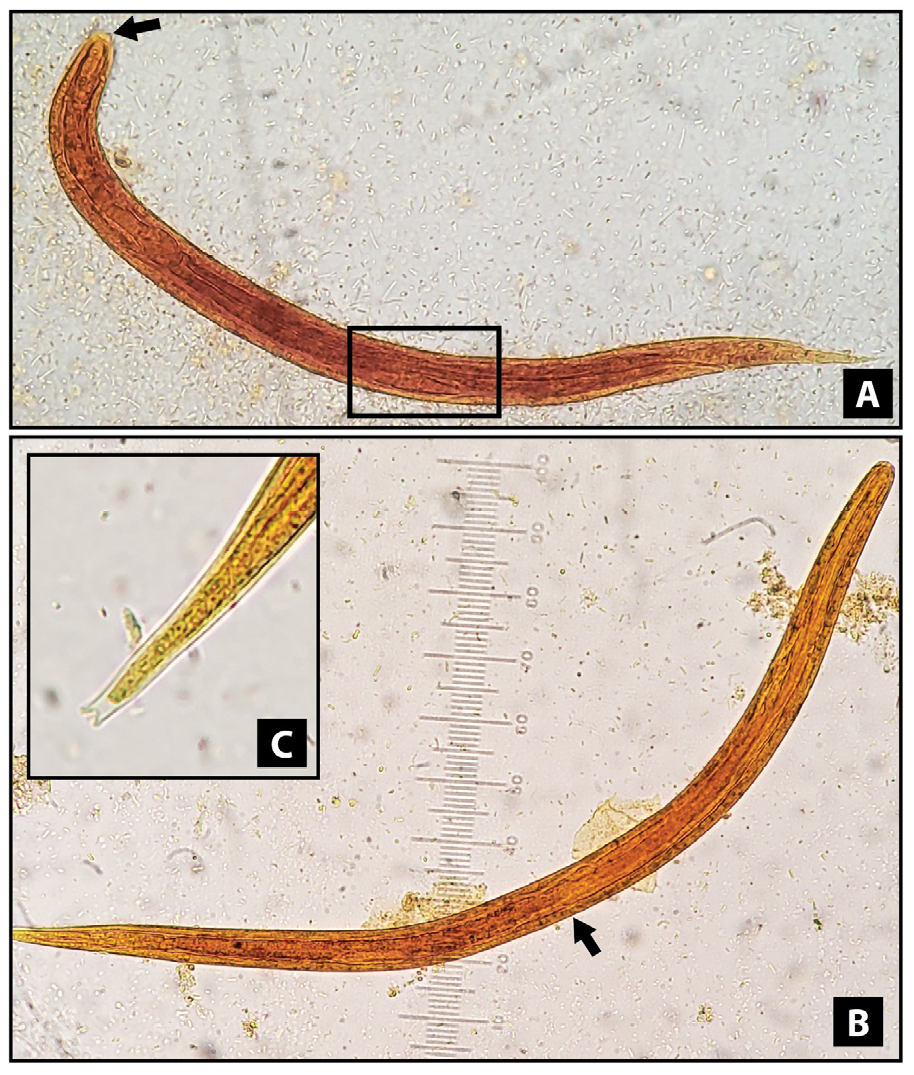
Results. The frequency of strongyloidiasis was 0.29% (112/38,085). It was higher with the modified Baermann technique (0.87%; 40/4,575) among male patients (0.44%;
70/15,758). Regarding the age, strongyloidiasis was higher in the 20-40 years old group (0.41%; 28/6,886) with direct smear and 41-61 years old (1.14%; 14/1,232) group with the modified Baermann technique. Among the factors associated with strongyloidiasis were age between 20 and 61 years old (PR=2.26, CI 95%=1.53-3.31), male patients (PR=2.34, CI 95%=1.60‑3.44), mucus (PR=1.86, CI 95%=1.22-2.83) and Charcot-Leyden crystals in stool (PR=8.47, CI 95%=5.14-13.96); watery stool (PR=2.39, CI 95%=1.55-3.68), and other helminthiases (PR=6.73, CI 95%=3.98-11.38). Associated factors to cases detected with the modified Baermann technique were outpatient consultation (PR=4.21, CI 95%=1.91-9.28) and formed stools (PR=3.99, CI 95%=1.94-8.19).
Conclusions. The modified Baermann technique increased the detection of strongyloidiasis almost four times. Most cases were distributed among male adults. The
cases diagnosed exclusively with the modified Baermann technique have differences from those with observed larvae in the direct smear. It is necessary to develop community-based population studies.
Downloads
References
Luvira V, Siripoon T, Phiboonbanakit D, Somsri K, Watthanakulpanich D, Dekumyoy P. Strongyloides stercoralis: A neglected but fatal parasite. Trop Med Infect Dis. 2022;7:310. https://doi.org/10.3390/tropicalmed7100310
Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, Greenaway C, et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9:468. https://doi.org/10.3390/pathogens9060468
Buonfrate D, Mena MA, Angheben A, Requena-Mendez A, Muñoz J, Gobbi F, et al. Prevalence of strongyloidiasis in Latin America: A systematic review of the literature. Epidemiol Infect. 2015;143:452-60. https://doi.org/10.1017/S0950268814001563
Ketzis J. Limitations to the adoption of a standardized Strongyloides stercoralis diagnostic method: Case study in the Caribbean. Acta Tropica. 2017;170:178-83. https://doi.org/10.1016/j.actatropica.2017.03.003
Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, et al. The laboratory diagnosis and follow up of strongyloidiasis: A systematic review. PLoS Negl Trop Dis. 2013;7:e2002. http://doi.org/10.1371/journal.pntd.0002002
Martins-de Paula F, de Mello-Malta F, Duarte-Marques P, Barnabé-Sitta R, Rebello-Pinho JR, Borges-Gryschek R, et al. Molecular diagnosis of strongyloidiasis in tropical areas: A comparison of conventional and real-time polymerase chain reaction with parasitological methods. Mem Inst Oswaldo Cruz. 2015;110:272-4. http://doi.org/10.1590/0074-02760140371
Asundi A, Beliavsky A, Liu X, Akaberi A, Schwarzer G, Bisoffi Z, et al. Prevalence of strongyloidiasis and schistosomiasis among migrants: A systematic review and metaanalysis. Lancet Glob Health. 2019;7:e236-48. https://doi.org/10.1016/S2214-109X(18)30490-X
Nutman T. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144:263-73. https://doi.org/10.1017/S0031182016000834
Czeresnia J, Weiss L. Strongyloides stercoralis. Lung. 2022;200:141-8. https://doi.org/10.1007/s00408-022-00528-z
Kaminsky RG. Guía de enfermedades parasitarias prevalentes en Honduras. Tegucigalpa, Honduras: Imprenta Honduras; 2019.
Mejia-Torres RE, Franco-Garcia D, Fontecha G, Hernandez-Santana A, Singh P, Mancero-Bucheli ST, et al. Prevalence and intensity of soil-transmitted helminthiasis, prevalence of malaria and nutritional status of school going children in Honduras. PLoS Negl Trop Dis. 2014;8:e3248. https://doi.org/10.1371/journal.pntd.0003248
Kaminsky RG. Estrongiloidiasis diseminada en una paciente viviendo con SIDA en Honduras. Rev Med Hondur. 2005;73:34-9.
Rivas-Godoy AF, Izaguirre-González AI, Maradiaga-Reyes EF, Bu-Figueroa E, García- Aguilar J. Estrongiloidiasis diseminada en una paciente con infección por el virus de la inmunodeficiencia humana (VIH). Med Int Mex. 2018;34:973-7. https://doi.org/10.24245/mim. v34i6.1978
García J, López W, Alger J, Matute ML, Kaminsky RG. Diagnóstico parasitológico de laboratorios clínicos públicos y privados de Tegucigalpa, Honduras: ¿Capacidad de Respuesta? Rev Med Hondur. 2014;82:148-54.
Kaminsky RG. Manual de Parasitología. Técnicas para laboratorio de Atención Primaria de Salud y para el diagnóstico de las Enfermedades Infecciosas Desatendidas. Tercera edición. Tegucigalpa: Imprenta Honduras; 2014.
Rugai E, Mattos T, Brisola AP. Nova Técnica para isolar larvas de nematoides das fezes, modificacão do metodo da Baermann. Rev Inst Adolfo Lutz. 1954;14:5-8. https://doi.org/10.53393/rial.1954.v14.33246
García J, Alger J. Método de Baermann y el diagnóstico de estrongiloidiasis. Rev Med Hondur. 2022;90:158. https://doi.org/10.5377/rmh.v90i2.14660
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022.
Stevenson M, Nunes ESwcfT, Heuer C, Marshall J, Sanchez J, Thornton R, et al. epiR: Tools for the analysis of epidemiological data. R package version 2.0.46. Fecha de consulta: 27 de enero del 2023. Available in: https://CRAN.R-project.org/package=epiR
Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: Global distribution and risk factors. PLoS Negl Trop Dis. 2013;7:e2288. https://doi.org/10.1371/journal.pntd.0002288
Kaminsky RG, Reyes-García S, Zambrano L. Unsuspected Strongyloides stercoralis infection in hospital patients with comorbidity in need of proper management. BMC Infect Dis. 2016;16:98. https://doi.org/10.1186/s12879-016-1424-3
Kaminsky RG. Actualización estadística sobre parasitismo intestinal. Resultados de laboratorio, Hospital Escuela, Honduras. Rev Med Hond. 2002;70:57-69.
Kaminsky RG. Aspectos epidemiológicos y conceptuales de parasitosis intestinales en el Hospital Regional de Tela, Honduras. Rev Med Hondur. 2012;80:90-5.
Kaminsky RG, Soto RJ, Campa A, Baum M. Intestinal parasitic infections and eosinophilia in a human immune deficiency virus-positive population in Honduras. Mem Inst Oswaldo Cruz. 2004;99:773-8. https://doi.org/10.1590/S0074-02762004000700020
Bouza-Mora L, Rodríguez-Masís D, Hernández-Chavarría F, Machado L. Strongyloides stercoralis en pacientes psiquiátricos. Rev Costarric Cienc Med. 2004;25:49-53.
Rivero-Rodríguez Z, Hernández A, Bracho A, Salazar S, Villalobos R. Prevalencia de microsporidios intestinales y otros enteroparásitos en pacientes con VIH positivo de Maracaibo, Venezuela. Biomédica. 2013;33:538-45. https://doi.org/10.7705/biomedica.v33i4.1468
Ortiz-Martínez S, Ramos-Rincón JM, Vásquez-Chasnamote ME, Gamboa-Paredes ON, Arista-Flores KM, Espinoza-Venegas LA, et al. Prevalence of strongyloidiasis in Peru: systematic review and meta-analysis. BMC Infect Dis. 2021;21:755. https://doi.org/10.1186/s12879-021-06441-9
Pereira Vieira Barreto NM, Brito Farias MM, Oliveira C de L, Almeida Costa Araujo W, Rios Grassi MF, Nascimento de Souza J, et al. Evaluation of Strongyloides stercoralis infection in patients with HTLV-1. Biomédica. 2022;42:31-40. https://doi.org/10.7705/biomedica.5888
Naves MM, Costa-Cruz JM. High prevalence of Strongyloides stercoralis infection among the elderly in Brazil. Rev Inst Med Trop Sao Paulo. 2013;55:309-13. https://doi.org/10.1590/S0036-46652013000500003
Rodrigues-Machado E, Costa-Cruz JM. Strongyloides stercoralis and other enteroparasites in children at Uberlândia City, State of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 1998;93:161-4. https://doi.org/10.1590/S0074-02761998000200004
Prasongdee T, Laoraksawong P, Kanarkard W, Kraiklang R, Sathapornworachai K, Naonongwai S, et al. An eleven-year retrospective hospital-based study of epidemiological data regarding human strongyloidiasis in northeast Thailand. BMC Infect Dis. 2017;17:627. https://doi.org/10.1186/s12879-017-2723-z
Jongwutiwes U, Waywa D, Silpasakorn S, Wanachiwanawin D, Suputtamongkol Y. Prevalence and risk factors of acquiring Strongyloides stercoralis infection among patients attending a tertiary hospital in Thailand. Pathog Glob Health. 2014;108:137-40. https://doi.org/10.1179/2047773214Y.0000000134
Chankongsin S, Wampfler R, Ruf MT, Odermatt P, Marti H, Nickel B, et al. Strongyloides stercoralis prevalence and diagnostics in Vientiane, Lao People’s Democratic Republic. Infect Dis Poverty. 2020;9:133. https://doi.org/10.1186/s40249-020-00750-y
Ashiri A, Rafiei A, Beiromvand M, Khanzadeh A, Alghasi A. Screening of Strongyloides stercoralis infection in high‑risk patients in Khuzestan Province, Southwestern Iran. Parasit Vectors. 2021;14:37. https://doi.org/10.1186/s13071-020-04549-6
Baker E, Ming D, Choudhury Y, Rahman S, Smith P, Muñoz J, et al. High Prevalence of Strongyloides among South Asian migrants in primary care-associations with eosinophilia and gastrointestinal symptoms. Pathogens. 2020;9:103. https://doi.org/10.3390/pathogens9020103
Winnicki W, Eder M, Mazal P, Mayer F, Sengölge G, Wagner L. Prevalence of Strongyloides stercoralis infection and hyperinfection syndrome among renal allograft recipients in Central Europe. Sci Rep. 2018;8:15406. https://doi.org/10.1038/s41598-018-33775-3
Guevara AG, Anselmi M, Bisoffi Z, Prandi R, Márquez M, Silva R, et al. Mapping the prevalence of Strongyloides stercoralis infection in Ecuador: A serosurvey. Am J Trop Med Hyg. 2020;102:346-9. https://doi.org/10.4269/ajtmh.19-0565
Requena-Méndez A, Salas-Coronas J, Salvador F, Gomez-Junyent J, Villar-Garcia J, Santin M, et al. High Prevalence of Strongyloidiasis in Spain: A Hospital-Based Study. Pathogens. 2020;9:109. https://doi.org/10.3390/pathogens9020107
Kaminsky RG. Validación del método de Baermann en vaso de sedimentación. Ciencia y Tecnología. 1992;14:12-8.
Kaminsky RG. Evaluation of Three Methods for Laboratory Diagnosis of Strongyloides stercoralis Infection. J Parasitol. 1993;79:277-80.
Fleitas PE, Travacio M, Martí-Soler H, Socías ME, López WR, Krolewiecki AJ. The Strongyloides stercoralis-hookworms association as a path to the estimation of the global burden of strongyloidiasis: A systematic review. PLoS Negl Trop Dis. 2020;14:e0008184. https://doi.org/10.1371/journal.pntd.0008184
Ueki S, Miyabe Y, Yamamoto Y, Fukuchi M, Hirokawa M, Spencer LA, et al. Charcot-Leyden crystals in eosinophilic inflammation: Active cytolysis leads to crystal formation. Curr Allergy Asthma Rep. 2019;19:35. https://doi.org/10.1007/s11882-019-0868-0. Erratum in: Curr Allergy Asthma Rep. 2019 Jul 13;19:38.
Secretaría de Salud de Honduras. Protocolo de manejo clínico del paciente adulto con COVID-19 según las etapas de la enfermedad en las Redes de Servicios de Salud. Tegucigalpa, Honduras; mayo 2020. Fecha de consulta: 8 de abril 2023. Disponible en: http://www.bvs.hn/COVID-19/Protocolo%20de%20Manejo%20Clinico%20del%20Paciente%20Adulto%20con%20COVID-19%20segun%20las%20etapas%20de%20la%20enfermedad%20en%20las%20redes%20de%20servicio%20de%20Salud%20Segunda%20Version.pdf
Some similar items:
- Leonardo Elías Ordóñez, Esther Sofía Angulo, Efficacy of ivermectin in the treatment of children parasitized by Strongyloides stercoralis. , Biomedica: Vol. 24 No. 1 (2004)
- Elba G. Rodríguez-Pérez, Alma Y. Arce-Mendoza, Roberto Saldívar-Palacios, Kevin Escandón-Vargas, Fatal Strongyloides stercoralis hyperinfection syndrome in an alcoholic diabetic patient from México , Biomedica: Vol. 40 No. Supl. 1 (2020): Mayo, Infecciones en el trópico
- Jorge García , Jackeline Alger, Ramón Jeremías Soto, Intestinal apicomplexan parasitoses among a hospital-based population in Honduras, 2013-2019 , Biomedica: Vol. 41 No. 4 (2021)
- Nilo Manoel Pereira Vieira Barreto , Marina Morena Brito Farias , Cíntia de Lima Oliveira, Weslei Almeida Costa Araujo , Maria Fernanda Rios Grassi , Joelma Nascimento de Souza , Beatriz Soares Jacobina , Márcia Cristina Aquino Teixeira, Bernardo Galvão-Castro, Neci Matos Soares, Evaluation of Strongyloides stercoralis infection in patients with HTLV-1 , Biomedica: Vol. 42 No. 1 (2022)
- María Isabel Giraldo, Nora Lizeth García, Jhon Carlos Castaño, Prevalence of intestinal helminths in dogs from Quindío Province. , Biomedica: Vol. 25 No. 3 (2005)
- Rodrigo Adán Medina-Pinto, Roger Iván Rodríguez-Vivas, Manuel Emilio Bolio-González, Zoonotic intestinal nematodes in dogs from public parks in Yucatán, México , Biomedica: Vol. 38 No. 1 (2018)
- Julián A. Fernández-Niño, Claudia I. Astudillo-García, Laura María Segura, Natalia Gómez, Ángela Skantria Salazar, Juan Hember Tabares, Cristian Andrés Restrepo, Miguel Ángel Ruiz, Myriam Consuelo López, Patricia Reyes, Profiles of intestinal polyparasitism in a community of the Colombian Amazon region , Biomedica: Vol. 37 No. 3 (2017)
- Álvaro Francisco Dulce-Villarreal, Angélica María Rojas-Bárcenas, José Danilo Jojoa-Ríos, José Fernando Gómez-Urrego, Human intestinal myiasis by Eristalis tenax in a child from the urban area of the municipality of Policarpa, Nariño, Colombia , Biomedica: Vol. 40 No. 4 (2020)
- Juan David Rojas, Mario Pereira , Bibiana Martínez , Julio César Gómez , Sonia Isabel Cuervo , Chagas disease reactivation after autologous stem cell transplant. Case report and literature review , Biomedica: Vol. 42 No. 2 (2022)
- Briana Beltrán, Dione Benjumea-Bedoya, Jackeline Alger , Factors affecting the tuberculosis program coverage at the first level of care in Honduras , Biomedica: Vol. 42 No. 2 (2022)
Copyright (c) 2023 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























