Eco-epidemiological aspects, natural detection and molecular identification of Leishmania spp. in Lutzomyia reburra, Lutzomyia barrettoi majuscula and Lutzomyia trapidoi
Abstract
Introduction: The province of Pichincha in Ecuador is an endemic area of cutaneous leishmaniasis, where anthropophilic sand flies with natural infection by Leishmania, have been reported as vectors. However, the role in transmission of zoophilic species has not been evaluated.
Objective: To evaluate natural infection by Leishmania in two zoophilic phlebotomine sand fly species, Lutzomyia reburra and Lu. barrettoi majuscula, and one anthropophilic species, Lu. trapidoi, as well as the endophagy and synanthropism of these species in the northwest of Pichincha.
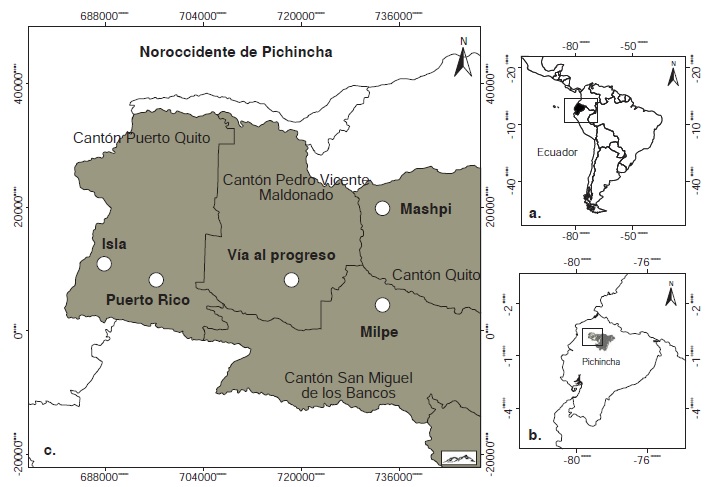
Materials and methods: Phlebotomines were collected using CDC light traps in different habitats and altitudes with presence of cutaneous leishmaniasis. Leishmania infection was detected using genomic DNA from females of the collected sand flies. We amplified the internal transcribed spacer gene of ribosomal RNA I (ITS1), the mitochondrial topoisomerase II gene (mtTOPOII), and the nuclear topoisomerase II gene (TopoII). Percentages of positivity for Leishmania, at spatio-temporal scale, proportion of endophagy and synanthropism index were calculated.
Results: Natural infection was determined for Le. amazonensis in Lu. reburra (9.5%) and Lu. b. majuscula (23.8%), while in Lu. trapidoi we detected Le. amazonensis, Le. brazilienis and Le. naiffi-lainsoni. Phlebotomines were asynanthropic and with low endophagy.
Conclusion: Natural infection with Le. amazonensis was recorded for the first time in Lu. reburra and Lu. b. majuscula, demonstrating the importance of zoophilic phlebotomines in the maintenance of the Leishmania transmission cycle in endemic foci.
Downloads
References
Alexander JB, Takaoka H, Eshita Y, Gómez EA, Hashiguchi Y. New records of phlebotomine sad flies (Diptera: Psychodidae) from Ecuador. Mem Inst Oswaldo Cruz.1992;87:123-30. https://doi.org/10.1590/S0074-02761 992000100019
LePont F, León R, Guerrini F, Gantier JC, Mouchet J, Echeverría R, et al. Leishmaniasis in Ecuador. 3. Lutzomyia trapidoi, vector of Leishmania panamensis. Ann Soc Belg Med Trop. 1994;74:23-8.
Jones LA, Cohnstaedt LW, Beati L, Terán R, León R, Munstermann LE. New records of phlebotomine sand flies (Diptera: Psychodidae) from Ecuador. Proc Entomol Soc Wash. 2010;112:47-53. https://doi.org/10.4289/0013-8797-112.1.47
Araújo P, Bersosa F, Carranco R, Granda V, Guerra P, Miranda N, et al. Evaluación preliminar de la diversidad de escarabajos (Insecta: Coleoptera) del Chocó ecuatoriano. Revista Politécnica, Biología. 2005;26:120-40.
Instituto Nacional de Estadística y Censos. Estadística demográfica en el Ecuador. Quito: INEC; 2010. p. 300.
Calvopina M, Armijos RX, Hashiguchi Y. Epidemiology of leishmaniasis in Ecuador: Current status of knowledge -- a review. Mem Inst Oswaldo Cruz. 2004;99:663-72. https://doi.org/10.1590/S0074-02762004000700001
Calvopiña M, Loor R, Lara F, Zambrano P, Hashiguchi Y. Prevalencia y formas clínicas de las leishmaniasis en el noroccidente de la provincia de Pichincha, Ecuador. Rev Fac Cien Med Quito. 2012;37:31-8.
Kato H, Uezato H, Gómez EA, Terayama Y, Calvopina M, Iwata H, et al. Establishment of a mass screening method of sand fly vectors for Leishmania infection by molecular biological methods. Am J Trop Med Hyg. 2007;77:324-9.
Santamaría E, Ponce N, Zipa Y, Ferro C. Presencia en el peridomicilio de vectores infectados con Leishmania (Viannia) panamensis en dos focos endémicos en el occidente de Boyacá, piedemonte del valle del Magdalena medio, Colombia. Biomédica. 2006;26:82-94. https://doi.org/10.7705/biomedica.v26i1.1503
Gómez EA, Kato H, Mimori T, Hashiguchi Y. Distribution of Lutzomyia ayacuchensis, the vector of Andean-type cutaneous leishmaniasis, at different altitudes on the Andean slope of Ecuador. Acta Trop. 2014;137:118-22. https://doi.org/10.1016/j.actatropica.2014.05.006
Kato H, Uezato H, Katakura K, Calvopiña M, Marco JD, Barroso PA, et al. Detection and identification of Leishmania species within naturally infected sand flies in the Andean areas of Ecuador by a polymerase chain reaction. Am J Trop Med Hyg. 2005;72:87-93.
Duque P, Vélez I, Morales M, Sierra D. Sand flies fauna involved in the transmission of cutaneous leishmaniasis in afro-Colombian and Amerindian communities of Chocó Pacific Coast of Colombia. Neotrop Entomol. 2004;33:255-64. https://doi.org/10.1590/S1519-566X2004000200018
Gómez EA, Kato H, Hashiguchi Y. Man-biting sand fly species and natural infection with the Leishmania pro-mastigote in leishmaniasis-endemic areas of Ecuador. Acta Trop. 2014;140:41-9. https://doi.org/10.1016/j.actatropica. 2014.07.003
Young DG, Duncan M. Guide to the identification and geographic distribution of Lutzomyia sandflies in México, the West Indies, Central and South America (Diptera: Psychodidae). Gainesville, Florida: Associated Publishers; 1994.
Ministerio del Ambiente de Ecuador. Línea base de deforestación del Ecuador Continental. Quito: Ministerio del Ambiente del Ecuador; 2012. p. 1-32.
Bejarano EE, Uribe S, Rojas W, Vélez ID. Phlebotomine sand flies (Diptera: Psychodidae) associated with the appearance of urban leishmaniasis in the city of Sincelejo, Colombia. Mem Inst Oswaldo Cruz. 2002;97:645-7. https://doi.org/10.1590/S0074-02762002000500010
Rebollar-Téllez E, Ramírez-Fraire A, Andrade-Narváez FJ. A two years study on vectors of cutaneous leishmaniasis. Evidence for sylvatic transmission cycle in the state of Campeche, México. Mem Inst Oswaldo Cruz. 1996;91:555-60. https://doi.org/10.1590/S0074-02761996000500004
Rodrigues W, Medeiros J, Julião GR, Ríos-Velásquez CM, Marialvac EF, Desmouliérec S, et al. Anthropic effects on sand fly (Diptera: Psychodidae) abundance and diversity in an Amazonian rural settlement, Brazil. Acta Trop. 2014;139:44-52. https://doi.org/10.1016/j.actatropica. 2014.06.017
Plisco P, Fuentes-Castillo T. Modelación de la distribución de especies y ecosistemas en el tiempo y en el espacio: una revisión de las nuevas herramientas y enfoques disponibles. Rev Geogr Norte Gd. 2011;48:61-79. https://doi.org/10.4067/S0718-34022011000100005
Quintana MG, Salomón OD, De Grosso MS. Distribution of phlebotomine sand flies (Diptera: Psychodidae) in a primary forest-crop interface, Salta, Argentina. J Med Entomol. 2010;47:1003-10.
Kato H, Gómez EA, Martini-Robles L, Muzzio J, Vélez L, Calvopiña M, et al. Geographic distribution of Leishmania species in Ecuador based on the cytochrome B gene sequence analysis. PLoS Negl Trop Dis. 2016;10:e0004844. https://doi.org/10.1371/journal.pntd.0004844
El Tai NO, El Fari M, Mauricio I, Miles MA, Oskam L, El Safi SH, et al. Leishmania donovani: Intraspecific polymorphisms of Sudanese isolates revealed by PCR-based analyses and DNA sequencing. Exp Parasitol. 2001;97:35-44. https://doi.org/10.1006/expr.2001.4592
Hijjawi N, Kanani KA, Rasheed M, Atoum M, Abdel-Dayem M, Irhimeh MR. Molecular diagnosis and identifi-cation of Leishmania species in Jordan from saved dry samples. Biomed Res Int. 2016;2016:6871739. https://doi.org/10.1155/2016/6871739
Freitas-Lidani KC, Messias-Reason IJ, Ishikawa EA. A comparison of molecular markers to detect Lutzomyia longipalpis naturally infected with Leishmania (Leishmania) infantum. Mem Inst Oswaldo Cruz. 2014;109:442-7. https://doi.org/10.1590/0074-0276130285
Uzcanga G, Lara E, Gutiérrez, Beaty D, Beske T, Teran R, et al. Nuclear DNA replication and repair in parasites of the genus Leishmania exploting differences to develop innovative therapeutic approaches. Crit Rev Microbiol. 2017; 43:156-77. https://doi.org/10.1080/1040841X.2016.1188758
Pommier Y. Drugging topoisomerases: Lessons and challenges. ACS Chem Biol. 2013;8:82-95. https://doi.org/10. 1021/cb300648v
Villacís B, Carrillo D. Estadística demográfica en el Ecuador: diagnóstico y propuesta. Quito: Instituto Nacional de Estadística y Censos; 2011. p. 1-74.
Instituto Nacional de Meteorología e Hidrología. Anuario Meteorológico N° 52-2012. Quito: INMH; 2012. p. 132.
Golczer G, Arrivillaga J. Modificación de un protocolo estándar de extracción de ADN para flebotominos pequeños (Phlebotominae: Lutzomyia). Rev Col Entomol. 2008;34: 199-202.
Echeverría-Fonseca G, Mera-Ruiz PA, Carrillo-Toro J, Rodríguez-Hidalgo R. A new DNA extraction protocol for screwworm fly Cochliomyia species (Diptera: Calliphoridae). Front Environ Sci. 2015;2:68. https://doi.org/10.3389/fenvs. 2014.00068
Higgins DG, Bleasby AJ, Fuchs R. Clustal V improved software for multiple sequence alignment. Comp Appl Biosci. 1992;8:189-191.
Saito N, Nei M. The neighbor-joining method a new method for reconstructing phylogenetic trees. Mol Biol Evol.1987;4:406-25. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Swofford DL. PAUP*. Phylogenetic Analysis Using Parsi-mony (*and other methods). Version 4.0b10. Suderland, MA: Sinauer association Inc; 2001. http://benedick.rutgers.edu/software-manuals/PAUP4-manual.pdf
Arrivillaga JC, Norris D, Feliciangeli MD, Lanzaro G. Phylogeography of the neotropical sand fly Lutzomyia longipalpis inferred from mitochondrial DNA sequences. Infect Genet Evol. 2002;2:83-95. https://doi.org/10.1016/S1567-1348(02)00087-4
Arrivillaga J, Mutebi JP, Piñango H, Norris D, Alexander, B, Feliciangeli MD, et al. The taxonomic status of genetically divergent populations of Lutzomyia longipalpis (Diptera: Psychodidae) based on the distribution of mitochondrial and isozyme variation. J Med Entomol. 2003;40:615-27.
Logan-Klumpler FJ, De Silva N, Boehme U, Rogers MB, Velarde G, McQuillan J, et al. GeneDB-an annotation database for pathogens. Nucl Acids Res. 2012;40:98-108. https://doi.org/10.1093/nar/gkr1032
Paiva BR, Oliveira AG, Dorval MEMC, Galati EAB, Malafronte RS. Species-specific identification of Leishmania in naturally infected sand flies captured in Mato Grosso do Sul state, Brazil. Acta Trop. 2010;115:126-30. https://doi.org/10.1016/j.actatropica.2010.02.013
Figueroa-Roa L, Linhares A. Synanthropy of Muscidae (Diptera) in the city of Valdivia, Chile. Neotrop Entomol. 2004;33:647-51. https://doi.org/10.1590/S1519-566X20040 00500016
SPSS, INC. Statistical Package for the Social Sciences. Version 15.0. Chicago, IL: SPSS; 2006.
Grimaldi G, Momen H, Naiff RD, McMahon-Pratt D, Barret TV. Characterization and classification of leishmanial parasite from humans, wild mammals, and sandflies in the Amazon region of Brazil. Am J Trop Med Hyg. 1981;44:645-61. https://doi.org/10.4269/ajtmh.1991.44.645
Ferreira AL, Sessa PA, Malta-Varejao JP, Falqueto A. Distribution of sand flies (Diptera:Pshychodidae) at different altitudes in a endemic region of American cutaneous leishmaniasis in the state of Espírito Santo, Brazil. Mem Inst Oswaldo Cruz. 2001;96:1061-7. https://doi.org/10.1590/S0074-02762001000800006
Armijos RX, Chico M, Cruz M, Guderian R, Kreutzer R, Berman J, et al. Human cutaneous leishmaniasis in Ecuador: Identification of parasites by enzyme electrophoresis. Am J Trop Med Hyg. 1990;42:424-8. https://doi.org/10.4269/ajtmh.1990.42.424
Bañuls AL, Guerrini F, Le Pont F, Barrera C, Espinel I, Guderian R, et al. Evidence for hybridization by multilocus enzyme electrophoresis and random amplified polymorphic DNA between Leishmania brasiliensis and L. panamensi/guyanensis in Ecuador. J Eukaryot Microbiol. 1997;44:408-11. https://doi.org/10.1111/j.1550-7408.1997.tb05716.x
Hashiguchi Y, Gómez E, de Coronel VV, Mimori T, Kawabata M, Furuya M, et al. Andean leishmaniasis in Ecuador caused by infection with Leishmania mexicana and L. major-like parasites. Am J Trop Med Hyg. 1991;44:205-17. https://doi.org/10.4269/ajtmh.1991.44.205
Da Silva ACT, Cupolillo E, Volpini AC, Almeida R, Romero GA. Species diversity causing human cutaneous leishmaniasis in Rio Branco, State of Acre, Brazil. Trop Med Inter Health. 2006;11:1388-98. https://doi.org/10.1111/j.1365-3156.2006.01695.x
Jennings Y, Almeida A. Phenotypic characterization of Leishmania spp. causing cutaneous leishmaniasis in the lower Amazon region, western Pará state, Brazil, reveals a putative hybrid parasite, Leishmania. Parasite. 2014;21:1-11. https://doi.org/10.1051/parasite/2014039
Aleixo JA, Nascimento ET, Monteiro GR, Fernandes MZ, Ramos AM, Wilson ME, et al. Atypical American visceral leishmaniasis caused by disseminated Leishmania amazonensis infection presenting with hepatitis and adeno-pathy. Trans R Soc Trop Med Hyg. 2006;100:79-82. https://doi.org/10.1016/j.trstmh.2005.06.025
Tolezano JE, Uliana SR, Taniguchi HH, Araújo MF, Barbosa JA, Barbosa JE, et al. The first records of Leishmania (Leishmania) amazonensis in dogs (Canis familiaris) diagnosed clinically as having canine visceral leishmaniasis from Araçatuba county, São Paulo state, Brazil. Vet Parasitol. 2007;149:280-4. https://doi.org/10.1016/j.vetpar.2007.07.008
Hoffmann AR, Navarro IT, Camargo VE Jr, Caldart ET, Breganó RM, Pereira PM. Leishmania amazonensis in dog with clinical diagnosis of visceral leishmaniasis in Paraná state, Brazil - a case report. Semina: Ciências Agrárias. 2012;33:3265-70.
Some similar items:
- Johana Marin, Daniel Urrea, Carlos Muskus, María Clara Echeverry, Ana María Mejía, Omar Triana, High-resolution melting analysis based on specific genomic regions: A promising tool for the diagnosis and typing of species causing cutaneous leishmaniasis in Colombia , Biomedica: Vol. 37 No. 4 (2017)
- Luis Alberto Cortés, Jhon James Fernández, Species of Lutzomyia involved in an urban focus of visceral and cutaneous leishmaniasis , Biomedica: Vol. 28 No. 3 (2008)
- Clemencia Ovalle-Bracho, Carolina Camargo, Yira Díaz-Toro, Marcela Parra-Muñoz, Molecular typing of Leishmania (Leishmania) amazonensis and species of the subgenus Viannia associated with cutaneous and mucosal leishmaniasis in Colombia: A concordance study , Biomedica: Vol. 38 No. 1 (2018)
- Diego Fernando Zea, Martín Prager, Roger Adrian Figueroa, María Consuelo Miranda, Mucosal complication of cutaneous leishmaniasis , Biomedica: Vol. 29 No. 1 (2009)
- Camilo Andrés Morales, Juliana Palacio, Gerzaín Rodríguez, Yenny Carolina Camargo, Zosteriform cutaneous leishmaniasis due to Leishmania (Viannia) panamensis and Leishmania (Viannia) braziliensis: Report of three cases , Biomedica: Vol. 34 No. 3 (2014)
- Ana M. Montalvo, Jorge Fraga, Ivón Montano, Lianet Monzote, Gert Van der Auwera, Marcel Marín, Carlos Muskus, Molecular identification of Leishmania spp. clinical isolates from Colombia based on hsp70 gene , Biomedica: Vol. 36 (2016): Suplemento 1, Microbiología médica
- Olga Lucía Cabrera, Laureano Mosquera, Erika Santamaría, Sand flies (Diptera: Psychodidae) of Guaviare Province, Colombia, with 4 new records for the country , Biomedica: Vol. 29 No. 1 (2009)
- Ana Margarita Montalvo, Lianet Monzote, Jorge Fraga, Ivón Montano, Carlos Muskus, Marcel Marín, Simonne De Donck, Iván Darío Vélez, Jean Claude Dujardin, PCR-RFLP and RAPD for typing neotropical Leishmania , Biomedica: Vol. 28 No. 4 (2008)
- Eduar Elías Bejarano, Diana Sierra, Iván Darío Vélez, New findings on the geographic distribution of the verrucarum group (Diptera: Psychodidae) in Colombia. , Biomedica: Vol. 23 No. 3 (2003)
- Marcel Marín, Yudy Alexandra Aguilar, José Robinson Ramírez, Omar Triana, Carlos Enrique Muskus, Molecular and immunological analyses suggest the absence of hydrophilic surface proteins in Leishmania (Viannia) panamensis , Biomedica: Vol. 28 No. 3 (2008)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























